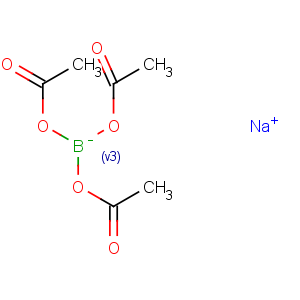
Title: Sodium Triacetoxyborohydride
CAS Registry Number: 56553-60-7
CAS Name: Sodium (
T-4)-tris(acetato-k
O)hydroborate(1-)
Molecular Formula: C6H10BNaO6
Molecular Weight: 211.94
Percent Composition: C 34.00%, H 4.76%, B 5.10%, Na 10.85%, O 45.29%
Literature References: Mild borohydride reagent. Prepn and use in reduction of aldehydes: G. W. Gribble, D. C. Ferguson,
J. Chem. Soc. Chem. Commun. 1975, 535. Reductive amination of aldehydes and ketones: A. F. Abdel-Magid
et al., J. Org. Chem. 61, 3849 (1996); L.-X. Yang, K. G. Hofer,
Tetrahedron Lett. 37, 6081 (1996); of amino acid esters: J. M. Ramanjulu, M. M. Joullié,
Synth. Commun. 26, 1379 (1996). Reductive alkylation of amines: Y. Han, M. Chorev,
J. Org. Chem. 64, 1972 (1999). Reduction of conjugated aldehydes: J. Singh
et al., Synth. Commun. 30, 1515 (2000). Stereoselective reduction of ketones: Y.-T. Liu
et al., Tetrahedron Lett. 45, 6097 (2004).
Use: Selective reducing agent in organic synthesis.