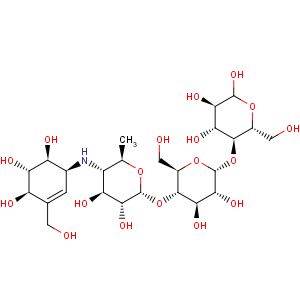
Title: Acarbose
CAS Registry Number: 56180-94-0
CAS Name: O-4,6-Dideoxy-4-[[[1
S-(1a,4a,5b,6a)]-4,5,6-trihydroxy-3-(hydroxymethyl)-2-cyclohexen-1-yl]amino]-a-D-glucopyranosyl-(1?4)-
O-a-D-glucopyranosyl-(1?4)-D-glucose
Synonyms: 4¢¢,6¢¢-dideoxy-4¢¢-[(1
S)-(1,4,6/5)-4,5,6-trihydroxy-3-hydroxymethyl-2-cyclohexenylamino]maltotriose
Manufacturers' Codes: Bay g 5421
Trademarks: Glucobay (Bayer); Prandase (Bayer); Precose (Bayer)
Molecular Formula: C25H43NO18
Molecular Weight: 645.60
Percent Composition: C 46.51%, H 6.71%, N 2.17%, O 44.61%
Literature References: Pseudotetrasaccharide containing an unsaturated cyclitol moiety. An a-glucosidase inhibitor that reduces sugar absorption in the gastrointestinal tract. Isoln from strains of
Actinoplanes: W. Frommer
et al., DE 2347782;
eidem, US 4062950 (1975, 1977 both to Bayer). Total synthesis: S. Ogawa, Y. Shibata,
Chem. Commun. 1988, 605;
eidem, Carbohydr. Res. 189, 309 (1989). Biosynthetic studies: U. Degwert
et al., J. Antibiot. 40, 855 (1987). Glucosidase inhibition studies: D. D. Schmidt
et al., Naturwissenschaften 64, 535 (1977); W. Puls
et al., ibid. 536. Use in treatment of diabetic adults: D. Sailor, G. Roder,
Arzneim.-Forsch. 30, 2182 (1980); H. Laube
et al., ibid. 1154. Long-term study in sulfonylurea-treated diabetics: H. Vierhapper
et al., Diabetologia 20, 586 (1981). Potential use in prophylaxis of dental caries: N. E. Fiehn, D. Moe,
Scand. J. Dent. Res. 90, 124 (1982). Review of pharmacodynamics, pharmacokinetics and therapeutic potential: S. P. Clissold, C. Edwards,
Drugs 35, 214-243 (1988).
Properties: Amorphous powder. [a]D18 +165° (c = 0.4 in water).
Optical Rotation: [a]D18 +165° (c = 0.4 in water)
Therap-Cat: Antidiabetic.
Keywords: Antidiabetic; a-Glucosidase Inhibitor.