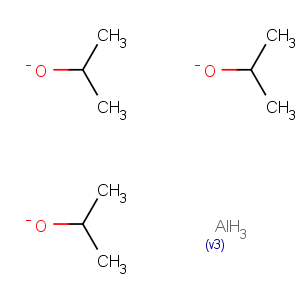
Title: Aluminum Isopropoxide
CAS Registry Number: 555-31-7
CAS Name: 2-Propanol aluminum salt
Synonyms: aluminum isopropylate
Molecular Formula: C9H21AlO3
Molecular Weight: 204.24
Percent Composition: C 52.93%, H 10.36%, Al 13.21%, O 23.50%
Line Formula: Al[OCH(CH3)2]3
Literature References: Prepd from aluminum and isopropyl alcohol in the presence of mercuric chloride: Young
et al., J. Am. Chem. Soc. 58, 100 (1936); by adding excess isopropyl alcohol to a benzene soln of AlCl3 at 6°: Teichner,
Compt. Rend. 237, 810 (1953). Forms trimers and tetramers: Shiner
et al., J. Am. Chem. Soc. 85, 2318 (1963); Oliver
et al., J. Inorg. Nucl. Chem. 31, 1609 (1969); Worrall,
J. Chem. Educ. 46, 510 (1969). Toxicity: Smyth
et al., Am. Ind. Hyg. Assoc. J. 30, 470 (1969).
Review: Whitaker in
Adv. Chem. Ser. 23, entitled "Metal-Organic Compounds," M. Sittig, Ed. (ACS, Washington DC, 1959) pp 184-189.
Properties: Hygroscopic white solid, mp 119°. Solidifies rather slowly after distillation. bp10 135°; bp7.5 131°; bp5.5 125.5°; bp2.5 113°; bp1.5 106°; bp0.5 94°. Sol in ethanol, isopropanol, benzene, toluene, chloroform, carbon tetrachloride, petroleum hydrocarbons. Decomposed by water. LD50 orally in rats: 11.3 g/kg (Smyth).
Melting point: mp 119°
Boiling point: bp10 135°; bp7.5 131°; bp5.5 125.5°; bp2.5 113°; bp1.5 106°; bp0.5 94°
Toxicity data: LD50 orally in rats: 11.3 g/kg (Smyth)
Use: Meerwein-Ponndorf reactions; alcoholysis and ester exchange; synthesis of higher alkoxides, chelates, and acylates; formation of aluminum soaps, formulation of paints; waterproofing finishes for textiles.