Title: Lithium Carbonate
CAS Registry Number: 554-13-2
Trademarks: Camcolit (Norgine); Carbolith (Valeant); Eskalith (GSK); Hypnorex (Sanofi-Synthelabo); Limas (Taisho); Liskonum (GSK); Lithane (Bayer); Lithicarb (Aspen); Lithobid (Solvay); Plenur (Ipsen); Priadel (Sanofi-Aventis); Quilonum-retard (GSK); Téralithe (Aventis)
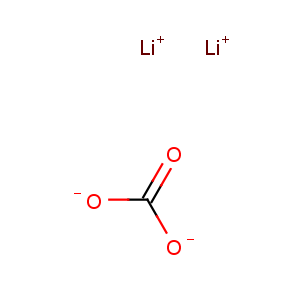
Molecular Formula: CLi2O3
Molecular Weight: 73.89
Percent Composition: C 16.25%, Li 18.79%, O 64.96%
Line Formula: Li2CO3
Literature References: Purification: Caley, Elving,
Inorg. Synth. 1, 1 (1939). Initial report of effect in mental illness: J. F. J. Cade,
Med. J. Aust. 2, 349 (1949). Early review of biology and pharmacology of lithium: M. Schou,
Pharmacol. Rev. 9, 17-58 (1957); of toxicity:
idem, Acta Pharmacol. Toxicol. 15, 70-84 (1958). Thermal behavior: A. Reisman,
J. Am. Chem. Soc. 80, 3558 (1958). Acute toxicity data: H. F. Smyth
et al., Am. Ind. Hyg. Assoc. J. 30, 470 (1969). Toxicological studies in mammals: Gralla, McIlhenny,
Toxicol. Appl. Pharmacol. 21, 428 (1972). Pharmacokinetics: J. S. Ku
et al., Int. J. Clin. Pharmacol. Ther. Toxicol. 25, 648 (1987). Clinical studies: J. Rybakowski
et al., Int. Pharmacopsychiatry 15, 86 (1980); B. F. Kjellman
et al., Acta Psychiatr. Scand. 62, 32 (1980). Review of development of use: G. Chouinard,
Union Med. Can. 109, 221-224, 226, 304 (1980). Comprehensive description: H. C. Stober,
Anal. Profiles Drug Subs. 15, 367-391 (1986).
Properties: White, light, alkaline powder. d 2.11. mp 720 ±1°. One gram dissolves in 78 ml cold, 140 ml boiling water; dissolved by dil acids. Practically insol in alcohol. LD50 orally in rats: 0.71 g/kg (Smyth).
Melting point: mp 720 ±1°
Density: d 2.11
Toxicity data: LD50 orally in rats: 0.71 g/kg (Smyth)
Use: In the production of glazes on ceramic and electrical porcelain.
Therap-Cat: Antimanic.
Keywords: Antimanic.