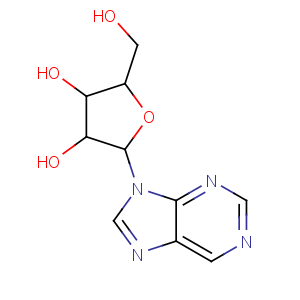
Title: Nebularine
CAS Registry Number: 550-33-4
CAS Name: 9-b-D-Ribofuranosyl-9
H-purine
Molecular Formula: C10H12N4O4
Molecular Weight: 252.23
Percent Composition: C 47.62%, H 4.80%, N 22.21%, O 25.37%
Literature References: Isoln from the mushroom
Clitocybe nebularis (Batsch.) Quel.,
Agaricaceae: Ehrenberg
et al., Sven. Kem. Tidskr. 58, 269 (1946); L?fgren
et al., Acta Chem. Scand. 8, 670 (1954); from a streptomycete: Isono, Suzuki,
J. Antibiot. 13A, 270 (1960).
In vitro toxicity towards sarcoma 180 cells, mouse embryonic fibroblasts and epithelial cells: J. J. Biescle
et al., Cancer 8, 87 (1955). Synthesis: Brown, Weliky,
J. Biol. Chem. 204, 1019 (1953); Fox
et al., J. Am. Chem. Soc. 80, 1669 (1958); Hashizume, Iwamura,
Tetrahedron Lett. 1966, 643;
eidem, J. Org. Chem. 33, 1796 (1968). Crystal structure: T. Takeda,
Acta Crystallogr. 30B, 825 (1974). Alternate syntheses: V. Nair, S. G. Richardson,
Tetrahedron Lett. 1979, 1181; P. K. Gupta, D. S. Bhakuni,
Indian J. Chem. 20B, 534 (1981). Toxicity studies: Truant, D'Amato,
Fed. Proc. 14, 391 (1955).
Properties: Small rhombohedra from ethyl methyl ketone + methanol, mp 181-182°; needles from methanol, mp 182-183°. [a]D25 -48.6° (H2O). uv max (0.1
N HCl): 262 nm (E1%1cm 232); (0.1
N NaOH): 263 nm (E1%1cm 361). Considerably sol in water (about 10%). Slightly sol in cold ethanol. Very slightly sol in acetone, ether, chloroform. Aq solns may be sterilized by boiling without decompn. LD50 in rats, guinea pigs (mg/kg): 220, 15 s.c. (Truant, D'Amato).
Melting point: mp 181-182°; mp 182-183°
Optical Rotation: [a]D25 -48.6° (H2O)
Absorption maximum: uv max (0.1
N HCl): 262 nm (E1%1cm 232); (0.1
N NaOH): 263 nm (E1%1cm 361)
Toxicity data: LD50 in rats, guinea pigs (mg/kg): 220, 15 s.c. (Truant, D'Amato)