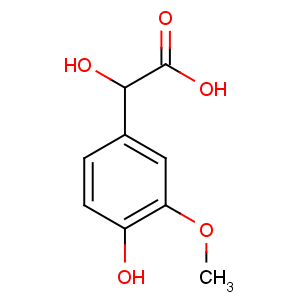
Title: Vanilmandelic Acid
CAS Registry Number: 55-10-7
CAS Name: a,4-Dihydroxy-3-methoxybenzeneacetic acid
Synonyms: 3-methoxy-4-hydroxymandelic acid; 4-hydroxy-3-methoxymandelic acid; VMA
Molecular Formula: C9H10O5
Molecular Weight: 198.17
Percent Composition: C 54.55%, H 5.09%, O 40.37%
Literature References: Catecholamine metabolite. Urine levels elevated in various pathologies. Misnamed
vanillinemandelic acid and
vanillylmandelic acid. Prepn from vanillin cyanohydrin: Gardner, Hibbert,
J. Am. Chem. Soc. 66, 608 (1944). Improved procedure: Shaw
et al., J. Org. Chem. 23, 30 (1958); E. F. Recondo, H. Rinderknecht,
ibid. 25, 2248 (1960); I. Goodman
et al., Biochem. Prep. 13, 75 (1971). Resolution: Armstrong
et al., Biochim. Biophys. Acta 25, 422 (1957). Determination in urine: T. C. Stewart, J. A. Freeman,
Vanilmandelic Acid & Catecholamine Determinations (Am. Soc. Clin. Pathol., Chicago, 1976) pp 1-81.
Derivative Type: DL-Form
Properties: Scales from ether + benzene, dec 131-133°; also reported as mp 134-135° (Goodman). uv max (0.1
N HCl): 230, 279 nm (e 6320, 2810); (0.1
N NaOH): 247, 285, 345 nm (e 6860, 3960, 630). Readily resinifies on heating or on prolonged exposure to air. Freely sol in water, acetone. Mod sol in ether, acetonitrile. Sparingly sol in benzene. Mass spectral data: T. R. Sharp,
Org. Mass Spectrom. 15, 381 (1980).
Melting point: mp 134-135° (Goodman)
Absorption maximum: uv max (0.1
N HCl): 230, 279 nm (e 6320, 2810); (0.1
N NaOH): 247, 285, 345 nm (e 6860, 3960, 630)
Derivative Type: L-Form
Properties: Crystals, dec 152°. [a]D22 +128° (c = 0.7).
Optical Rotation: [a]D22 +128° (c = 0.7)
Derivative Type: D-Form
Properties: Crystals, dec 152°. [a]D23 -131°.
Optical Rotation: [a]D23 -131°