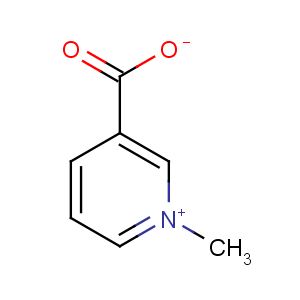
Title: Trigonelline
CAS Registry Number: 535-83-1
CAS Name: 3-Carboxy-1-methylpyridinium inner salt
Synonyms: nicotinic acid
N-methylbetaine; coffearine; caffearine; gynesine; trigenolline
Molecular Formula: C7H7NO2
Molecular Weight: 137.14
Percent Composition: C 61.31%, H 5.14%, N 10.21%, O 23.33%
Literature References: In seeds of
Trigonella foenumgraecum L.,
Leguminosae, in coffee beans, in seeds of
Strophanthus spp,
Apocynaceae and of
Cannabis sativa L.,
Moraceae, in seeds of many other plants; also in sea urchin,
Arabacia pustulosa, and in jellyfish,
Velella spirans. Excreted in urine after taking nicotinic acid: Ackermann,
Z. Biol. 59, 17 (1912). Isoln from normal urine: Linnewah, Renwein,
Z. Physiol. Chem. 207, 48 (1932);
209, 110 (1932). Syntheses: Turnau,
Monatsh. Chem. 26, 551 (1905); Sarett
et al., J. Biol. Chem. 135, 483 (1940); Green, Tong,
J. Am. Chem. Soc. 78, 4896 (1956); Kosower, Patton,
J. Org. Chem. 26, 1318 (1961). Toxicity study: Brazda, Coulson,
Proc. Soc. Exp. Biol. Med. 62, 19 (1946).
Derivative Type: Monohydrate
Properties: Crystals from ethanol, mp 230-233°. Salty taste. Very sol in water; sol in alcohol. Practically insol in ether, chloroform. LD50 s.c. in rats: 5.0 g/kg (Brazda, Coulson).
Melting point: mp 230-233°
Toxicity data: LD50 s.c. in rats: 5.0 g/kg (Brazda, Coulson)
Derivative Type: Hydrochloride
Molecular Formula: C7H7NO2.HCl
Molecular Weight: 173.60
Percent Composition: C 48.43%, H 4.64%, N 8.07%, O 18.43%, Cl 20.42%
Properties: Crystals from 90% alcohol, mp 258-259°. Very sol in water; slightly in alcohol. Practically insol in ether, benzene.
Melting point: mp 258-259°