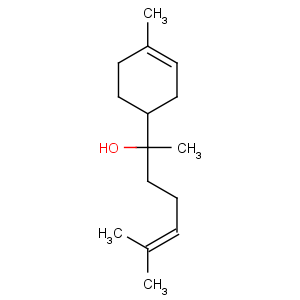
Title: a-Bisabolol
CAS Registry Number: 515-69-5
CAS Name: (a
R,1
R)-
rel-a,4-Dimethyl-a-(4-methyl-3-pentenyl)-3-cyclohexene-1-methanol
Synonyms: 6-methyl-2-(4-methyl-3-cyclohexen-1-yl)-5-hepten-2-ol; 1-methyl-4-(1,5-dimethyl-1-hydroxyhex-4(5)-enyl)cyclohexen-1
Trademarks: Camilol (Maybrook); Dragosantol (Dragoco); Hydagen B (Henkel)
Molecular Formula: C15H26O
Molecular Weight: 222.37
Percent Composition: C 81.02%, H 11.79%, O 7.19%
Literature References: Fragrant sesquiterpene isolated from the essential oils of a variety of plants, shrubs and trees. Both isomers occur in nature; the (-)-form is the most widespread and is an active anti-inflammatory component of chamomile. The (+) and (-)-epi isomers are also naturally occurring. Isoln of (-)-form from
Matricaria chamomilla: F. Sorm
et al., Collect. Czech. Chem. Commun. 16, 626 (1951); of (+)-form from Populus balsamifera:
idem et al., Chem. Listy 46, 364 (1952). Synthesis of racemic mixture: L. Ruzicka, M. Liguori,
Helv. Chim. Acta 15, 3 (1932); C. D. Gutsche
et al., Tetrahedron 24, 859 (1968). Enantioselective synthesis of (-)-form: H. Nemoto
et al., Tetrahedron Lett. 34, 4939 (1993). Separation of four stereoisomers: K. Günther
et al., Fresenius J. Anal. Chem. 345, 787 (1993). Pharmacology and toxicology of (-)-form: V. Jakovlev, A. von Schlichtegroll,
Arzneim.-Forsch. 19, 615 (1969); S. Habersang
et al., Planta Med. 37, 115 (1979). Comparative pharmacology of racemate and enantiomers: V. Jakovlev
et al., ibid. 35, 125 (1979). Determn by HPLC in chamomile extracts: R. Herrmann,
Dtsch. Apoth. Ztg. 36, 1797 (1982); by GC in cosmetic products: D. Andre
et al., Int. J. Cosmet. Sci. 13, 137 (1991). Evaluation of use as dermal penetration enhancer: R. Kadir, B. W. Barry,
Int. J. Pharm. 70, 87 (1991). Review of anti-inflammatory activity and use in cosmetics: N. Jellinek,
Parfums Cosmet. Aromes 57, 55-57 (1984); of absolute configuration studies: G. W. O'Donnell, M. D. Sutherland,
Aust. J. Chem. 42, 2021-2034 (1989).
Properties: bp12 155-157°. d423 0.9223.
nD23 1.4917. Also reported as colorless oil, bp1.5 128-130° (Gutsche). Miscible with alcohols, oils and lipophilic substances.
Boiling point: bp12 155-157°; bp1.5 128-130° (Gutsche)
Index of refraction: nD23 1.4917
Density: d423 0.9223
Derivative Type: (-)-Form
CAS Registry Number: 23089-26-1
Synonyms: (a
S,1
S)-a-Bisabolol; levomenol
Properties: bp12 153°. d20 0.9211.
nD20 1.4936. [a]D -55.7°. Also reported as [a]D20 -51.02° (Günther). LD50 in mice, rats (mg/kg): 11350, 14850 orally (Jakovlev, von Schlichtegroll). LD50 in male, female mice, rats (ml/kg): 15.2, 15.0, 14.9, 15.6 orally (Habersang).
Boiling point: bp12 153°
Optical Rotation: [a]D -55.7°; [a]D20 -51.02° (Günther)
Index of refraction: nD20 1.4936
Density: d20 0.9211
Toxicity data: LD50 in male, female mice, rats (ml/kg): 15.2, 15.0, 14.9, 15.6 orally (Habersang)
Derivative Type: (+)-Form
CAS Registry Number: 23178-88-3
Synonyms: (a
R,1
R)-a-Bisabolol
Properties: bp1.0 120-122°. d20 0.9213.
nD20 1.4919. [a]D20 +51.7°. Also reported as [a]D20 +57.04° (Günther).
Boiling point: bp1.0 120-122°
Optical Rotation: [a]D20 +51.7°; [a]D20 +57.04°
Index of refraction: nD20 1.4919
Density: d20 0.9213
Derivative Type: (-)-Epi-a-bisabolol
CAS Registry Number: 78148-59-1
Synonyms: (a
R,1
S)-a-Bisabolol; anymol
Literature References: Isoln from
Myoporum crassifolium: K. G. O'Brien
et al., Aust. J. Chem. 6, 166 (1953).
Properties: [a]D20 -67.6°. Also reported as [a]D20 -67.48° (Günther).
Optical Rotation: [a]D20 -67.6°; [a]D20 -67.48°
Derivative Type: (+)-Epi-a-bisabolol
CAS Registry Number: 76738-75-5
Synonyms: (a
S,1
R)-a-Bisabolol
Literature References: Isoln from
Salvia stenophylla: E. J. Brunke, F. J. Hammerschmidt,
Proc. 15th Int. Symp. Essent. Oils Aromat. Plants 1985, 145.
Properties: [a]D20 +67.4°. Also reported as [a]D20 +70.11° (Günther).
Optical Rotation: [a]D20 +67.4°; [a]D20 +70.11°
Use: In cosmetics.
Therap-Cat: Anti-inflammatory.
Keywords: Anti-inflammatory (Nonsteroidal).