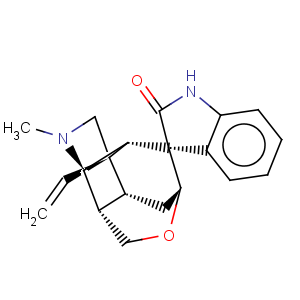
Title: Gelsemine
CAS Registry Number: 509-15-9
Molecular Formula: C20H22N2O2
Molecular Weight: 322.40
Percent Composition: C 74.51%, H 6.88%, N 8.69%, O 9.93%
Literature References: CNS stimulant from roots and rhizome of
Gelsemium sempervirens (L.) Ait.,
Loganiaceae. Isoln: Gerrard,
Pharm. J. 13, 641 (1883); Moore,
J. Chem. Soc. 97, 2223 (1910);
99, 1231 (1911); Sayre, Watson,
J. Am. Pharm. Assoc. 8, 708 (1919); Chou,
Chin. J. Physiol. 5, 131 (1931),
C.A. 25, 40856 (1931); Schwarz, Marion,
Can. J. Chem. 31, 958 (1953). Structure: Conroy, Chakrabarti,
Tetrahedron Lett. 1959 (4), 6; Lovell
et al., ibid. 1; Roe, Gates,
Tetrahedron 11, 148 (1960). NMR spectroscopic study: Y. Schun, G. A. Cordell,
J. Nat. Prod. 48, 969 (1985). Partial syntheses: W. E. Earley
et al., Tetrahedron Lett. 29, 3781, 3785 (1988).
Properties: Crystals from acetone, mp 178°.
Poisonous! [a]D20 +13° (c = 1.2 in chloroform). pKa 7.75 in 80% methylcellosolve. uv max (methanol): 210, 252, 280 nm (log e 4.50, 3.87, 3.15). Slightly sol in water; sol in alcohol, benzene, chloroform, ether, acetone, dilute acids.
Melting point: mp 178°
pKa: pKa 7.75 in 80% methylcellosolve
Optical Rotation: [a]D20 +13° (c = 1.2 in chloroform)
Absorption maximum: uv max (methanol): 210, 252, 280 nm (log e 4.50, 3.87, 3.15)
Derivative Type: Hydrochloride
Molecular Formula: C20H23ClN2O2
Molecular Weight: 358.86
Percent Composition: C 66.94%, H 6.46%, Cl 9.88%, N 7.81%, O 8.92%
Properties: Prisms from methanol + ether, mp 326°. [a]D +5° (c = 1.072 in water). Sol in water, slightly sol in alcohol.
Melting point: mp 326°
Optical Rotation: [a]D +5° (c = 1.072 in water)