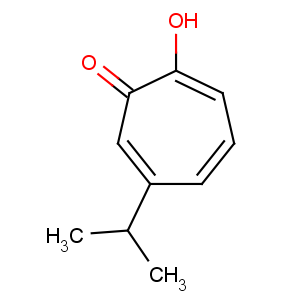
Title: b-Thujaplicin
CAS Registry Number: 499-44-5
CAS Name: 2-Hydroxy-4-(1-methylethyl)-2,4,6-cycloheptatrien-1-one
Synonyms: hinokitiol; 4-isopropyltropolone
Molecular Formula: C10H12O2
Molecular Weight: 164.20
Percent Composition: C 73.15%, H 7.37%, O 19.49%
Literature References: Tropolone derivative found in the heartwood of cupressaceous plants including western red cedar, eastern white cedar, and hiba; b-thujaplicin antimicrobial activity contributes to the decay-resistance of these trees. Isoln from western red cedar,
Thuja plicata D. Don: H. Erdtman, J. Gripenberg,
Nature 161, 719 (1948); and characterization: A. B. Anderson, J. Gripenberg,
Acta Chem. Scand. 2, 644 (1948). Synthesis: W. von E. Doering, L. H. Knox,
J. Am. Chem. Soc. 75, 297 (1953). Biosynthesis in
Cupressus lusitanica: J. Zhao, K. Sakai,
J. Exp. Bot. 54, 647 (2003). HPLC determn in cosmetics: M. Endo
et al., J. Chromatogr. 455, 430 (1988). CZE determn in aqueous solns: L. Dyrskov
et al., J. Agric. Food Chem. 52, 1452 (2004). Antibacterial activity in atopic dermatitis: Y. Arima
et al., J. Antimicrob. Chemother. 51, 113 (2003).
Properties: mp 52-52.5° (Erdtman); also reported as mp 50-51° (Doering). uv max (isooctane): 236, 322, 353 nm (log e 4.37, 3.72, 3.66).
Melting point: mp 52-52.5° (Erdtman); mp 50-51° (Doering)
Absorption maximum: uv max (isooctane): 236, 322, 353 nm (log e 4.37, 3.72, 3.66)
Use: Antibacterial additive in foods, cosmetics, eye drops and toothpaste.