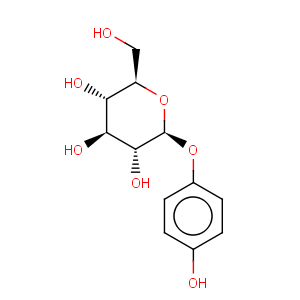
Title: Arbutin
CAS Registry Number: 497-76-7
CAS Name: 4-Hydroxyphenyl-b-D-glucopyranoside
Synonyms: hydroquinone-b-D-glucopyranoside; hydroquinone glucose; arbutoside; ursin
Molecular Formula: C12H16O7
Molecular Weight: 272.25
Percent Composition: C 52.94%, H 5.92%, O 41.14%
Literature References: Naturally occurring glycoside of hydroquinone,
q.v., found in the bark and leaves of various plants, usually occurring together with methylarbutin. Principal antibacterial constituent of the traditional medicine, uva ursi,
q.v. Isoln from bearberry leaves
(Arctostaphylos uva-ursi Spreng.,
Ericaceae): A. Kawalier,
Ann. 82, 241 (1852); from leaves of blueberry, cranberry, and pear
(Pyrus communis L.,
Rosaceae): G. Urban, M. Rogowski,
Arch. Exp. Pathol. Pharmakol. 211, 194 (1950). Synthesis: C. Mannich,
Arch. Pharm. 250, 547 (1912); A. Robertson, R. B. Waters,
J. Chem. Soc. 1930, 2729. Production by plant cell culture: S. Inomata
et al., Appl. Microbiol. Biotechnol. 36, 315 (1991). Physical properties: E. Lindpainter,
Arch. Pharm. 277, 398 (1940). Determn in drug prepns: L. Kraus,
Pharmazie 19, 41 (1964); E. Kenndler
et al., J. Chromatogr. 514, 383 (1990). Inhibition of human melanin synthesis
in vitro: K. Maeda, M. Fukuda,
J. Pharmacol. Exp. Ther. 276, 765 (1996). Review of human exposure: P. J. Deisinger
et al., J. Toxicol. Environ. Health 47, 31-46 (1996).
Properties: Occurs as the monohydrate. Colorless elongated prisms from moist ethyl acetate, mp 199° after sintering at 163-164° (Robertson, Waters). Also reported as unstable form, mp 165°; stable form, mp 199.5-200° (Lindpaintner). [a]D20 -60.3° (in water). Sol in water and in alc.
Melting point: mp 199° after sintering at 163-164° (Robertson, Waters); unstable form, mp 165°; stable form, mp 199.5-200° (Lindpaintner)
Optical Rotation: [a]D20 -60.3° (in water)
Derivative Type: Methylarbutin
CAS Registry Number: 6032-32-2
CAS Name: 4-Methoxyphenyl-b-D-glucopyranoside
Synonyms: methylarbutoside
Molecular Formula: C13H18O7
Molecular Weight: 286.28
Percent Composition: C 54.54%, H 6.34%, O 39.12%
Properties: Crystallizes from water as the monohydrate, mp 158-160°; solidifies and melts again at 175° (Mannich). Also reported as unstable form, mp 160.5°; stable form, mp 176° (Lindpaintner). [a]D20 -60.66° (in water). Sol in hot water or alcohol; slightly sol in ether.
Melting point: mp 158-160°; solidifies and melts again at 175° (Mannich); unstable form, mp 160.5°; stable form, mp 176° (Lindpaintner)
Optical Rotation: [a]D20 -60.66° (in water)
Use: In cosmetics as skin lightening agent.