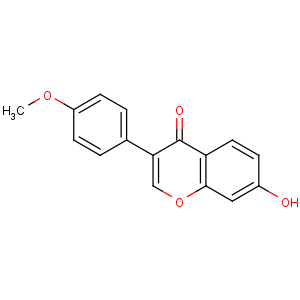
Title: Formononetin
CAS Registry Number: 485-72-3
CAS Name: 7-Hydroxy-3-(4-methoxyphenyl)-4
H-1-benzopyran-4-one
Synonyms: 7-hydroxy-4¢-methoxyisoflavone; biochanin B; formononetol; neochanin
Molecular Formula: C16H12O4
Molecular Weight: 268.26
Percent Composition: C 71.64%, H 4.51%, O 23.86%
Literature References: Isoln from soy-bean meal
(Soja hispida): Walz,
Ann. 489, 118 (1931); from clover species
Trifolium subterraneum L. and
T. pratense L.,
Leguminosae in which it is the major estrogenic factor: Bradbury, White,
J. Chem. Soc. 1951, 3447; Bate-Smith, Swain,
Chem. Ind. (London) 1953, 1127. Identity with biochanin B: Bose,
J. Sci. Ind. Res. 15B, 325 (1956). Structure: Baker
et al., J. Chem. Soc. 1933, 274. Synthesis: Wessely
et al., Ber. 66, 685 (1933); Kagal
et al., Tetrahedron Lett. 1962, 593. Biological half-life in
Cicer arietinum L.,
Papilionatae: N. Amrhein, E. Diederich,
Naturwissenschaften 67, 40 (1980). HPLC analysis: R. E. Carlson,
J. Chromatogr. 198, 193 (1980). 13C-NMR study: H. C. Jha
et al., Can. J. Chem. 58, 1211 (1980). Mutagenicity study: R. M. Bartholomew
et al., Mutat. Res. 78, 317 (1980).
Properties: Needles from alcohol, mp 258°. uv max (ethanol): 250, 300 nm (e 27440, 11240).
Melting point: mp 258°
Absorption maximum: uv max (ethanol): 250, 300 nm (e 27440, 11240)
Derivative Type: 7-Glucoside
Synonyms: Ononin; 4¢-methyldaidzin
Molecular Formula: C22H22O9
Molecular Weight: 430.40
Percent Composition: C 61.39%, H 5.15%, O 33.46%
Literature References: From
Ononis spinosa L.,
Leguminosae: Hlasiwetz,
J. Prakt. Chem. 65, 415 (1855). Synthesis: Farkas, Varady,
Ber. 92, 819 (1959).
Properties: Needles from water, C22H22O9.H2O, mp 210-214°. When anhydr, dec 245°. [a]D25 -24.2° (pyridine). Sol in alcohol; slightly sol in water, ether.
Melting point: mp 210-214°
Optical Rotation: [a]D25 -24.2° (pyridine)