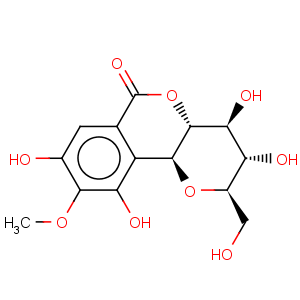
Title: Bergenin
CAS Registry Number: 477-90-7
CAS Name: 3,4,4a,10b-Tetrahydro-3,4,8,10-tetrahydroxy-2-(hydroxymethyl)-9-methoxypyrano[3,2-
c][2]benzopyran-6(2
H)-one
Synonyms: 4-methoxy-2-[tetrahydro-3,4,5-trihydroxy-6-(hydroxymethyl)pyran-2-yl]-a-resorcylic acid d-lactone; bergenit; vakerin; ardisic acid B; cuscutin; peltophorin
Molecular Formula: C14H16O9
Molecular Weight: 328.27
Percent Composition: C 51.22%, H 4.91%, O 43.86%
Literature References: From root of
Saxifraga (Bergenia) crassifolia L., and from rhizome of
S. sibirica L.
Saxifragaceae: Morelle,
Compt. Rend. 93, 646 (1881);
Ber. 14, 2694 (1881); Ssadikow, Guthner,
Biochem. Z. 190, 340 (1927); Tschitschibabin
et al., Ann. 469, 93 (1929). Identity with vakerin: Carruthers
et al., Chem. Ind. (London) 1957, 76. Identity with ardisic acid B: Hung, Chu,
C.A. 52, 15827h (1958). Identity with cuscutin: Jain, Mishra,
Indian J. Chem. 1, 499 (1963). Identity with peltophorin: Joshi, Kamat,
Naturwissenschaften 56, 89 (1969). Structure: Posternak, Dürr,
Helv. Chim. Acta 41, 1159 (1958); Fujise
et al., Bull. Chem. Soc. Jpn. 32, 97 (1959). Synthesis and structure: Hay, Haynes,
J. Chem. Soc. 1958, 2231. Biosynthesis: Wenkert,
Chem. Ind. (London) 1959, 906.
Properties: Crystals from methanol, mp 238°. uv max: 275, 220 nm (log e 3.92, 4.42). [a]D18 -37.7° (c = 1.96 in ethanol); [a]D24 -45.3° (c = 0.51 for anhydr in water). Freely sol in water; sol in alcohol.
Melting point: mp 238°
Optical Rotation: [a]D18 -37.7° (c = 1.96 in ethanol); [a]D24 -45.3° (c = 0.51 for anhydr in water)
Absorption maximum: uv max: 275, 220 nm (log e 3.92, 4.42)
Derivative Type: Monohydrate
Properties: Crystals from water, mp 140°. Slightly sol in water, freely in alcohol.
Melting point: mp 140°
Status: This monograph has been retired and is no longer subject to revision or update.