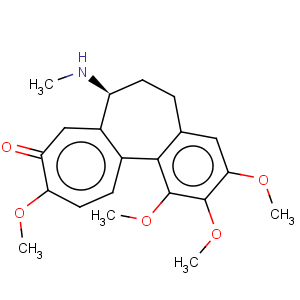
Title: Demecolcine
CAS Registry Number: 477-30-5
CAS Name: 6,7-Dihydro-1,2,3,10-tetramethoxy-7-(methylamino)benzo[
a]heptalen-9(5
H)-one
Synonyms: N-deacetyl-
N-methylcolchicine;
N-methyl-
N-desacetylcolchicine; colchamine; Santavy's substance F; colcemid
Molecular Formula: C21H25NO5
Molecular Weight: 371.43
Percent Composition: C 67.91%, H 6.78%, N 3.77%, O 21.54%
Literature References: Antitumor alkaloid; depolymerizes microtubules and inhibits spindle formation during metaphase. Isoln from
Colchicum autumnale L.,
Liliaceae: Santavy,
Pharm. Acta Helv. 25, 248 (1950); Santavy, Reichstein,
Helv. Chim. Acta 33, 1606 (1950); Schlittler, Uffer,
DE 936268 (1955 to Ciba),
C.A. 53, 1396 (1959). Structure: Santavy
et al., Helv. Chim. Acta 36, 1319 (1953). Synthesis: Uffer
et al., ibid. 37, 18 (1954); H. G. Capraro, A. Brossi,
ibid. 62, 965 (1979). Effect on the mitotic cycle: C. L. Rieder, R. E. Palazzo,
J. Cell Sci. 102, 387 (1992). Use in oocyte enucleation: E. Ibá?ez
et al., Biol. Reprod. 68, 1249 (2003).
Properties: Pale yellow prisms from ethyl acetate + ether, mp 186°. [a]D20 -129.0° (c = 1 in chloroform). uv max (alc): 245, 355 nm (log e 4.55, 4.24). Basic reaction. Soluble in acidified water, in alcohol, ether, chloroform, benzene.
Melting point: mp 186°
Optical Rotation: [a]D20 -129.0° (c = 1 in chloroform)
Absorption maximum: uv max (alc): 245, 355 nm (log e 4.55, 4.24)
Use: Cell synchronization agent; for chromosome visualization; to induce oocyte enucleation for somatic cell cloning.