References of 5,8-dihydroxy-2-methoxy-6-methyl-7-(2-oxopropyl)naphthalene-1,4-dione
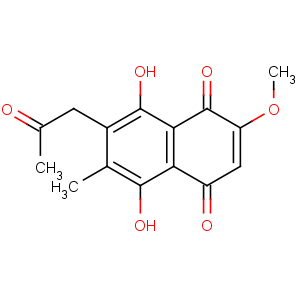
Title: Javanicin
CAS Registry Number: 476-45-9
CAS Name: 5,8-Dihydroxy-6-methoxy-2-methyl-3-(2-oxopropyl)-1,4-naphthalenedione
Synonyms: 3-acetonyl-5,8-dihydroxy-6-methoxy-2-methyl-1,4-naphthoquinone
Molecular Formula: C15H14O6
Molecular Weight: 290.27
Percent Composition: C 62.07%, H 4.86%, O 33.07%
Literature References: Antibiotic substance produced by
Fusarium javanicum: Arnstein, Cook,
J. Chem. Soc. 1947, 1021. Prepd by reduction of fusarubin: Ruelius, Gauhe,
Ann. 569, 38 (1950). Structure: Birch, Donovan,
Chem. Ind. (London) 1954, 1047; Whalley,
ibid. 1958, 131; Hardegger
et al., Helv. Chim. Acta 47, 2027 (1964).
Properties: Red crystals with a coppery luster from ethanol, decomp 207.5-208°. Absorption max (alc): 303, 305 nm (log e 3.97, 3.90); in chloroform: 307, 510 nm (log e 3.99, 3.86).
Absorption maximum: Absorption max (alc): 303, 305 nm (log e 3.97, 3.90); in chloroform: 307, 510 nm (log e 3.99, 3.86)
Derivative Type: Diacetyljavanicin
Molecular Formula: C19H18O8
Molecular Weight: 374.34
Percent Composition: C 60.96%, H 4.85%, O 34.19%
Properties: Needles from dil acetone, dec 207-208°. Absorption max (alc): 221, 290, 426 nm (log e 4.57, 4.17, 3.79).
Absorption maximum: Absorption max (alc): 221, 290, 426 nm (log e 4.57, 4.17, 3.79)