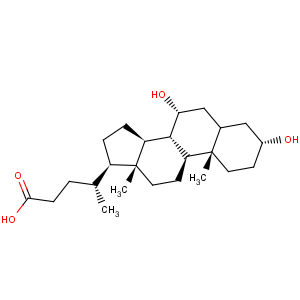
Title: Chenodiol
CAS Registry Number: 474-25-9
CAS Name: (3a,5b,7a)-3,7-Dihydroxycholan-24-oic acid
Synonyms: 3a,7a-dihydroxy-5b-cholanic acid; anthropodesoxycholic acid; gallodesoxycholic acid; 17b-(1-methyl-3-carboxypropyl)etiocholane-3a,7a-diol; chenic acid; chenodeoxycholic acid; CDC
Trademarks: Chendol (CP Pharm.); Chenocol (Astellas); Chenofalk (Falk); Chenossil (Sanofi-Aventis); Cholanorm (Grñenthal); Fluibil (Zambon)
Molecular Formula: C24H40O4
Molecular Weight: 392.57
Percent Composition: C 73.43%, H 10.27%, O 16.30%
Literature References: A major bile acid in many vertebrates, occurring as the
N-glycine and/or
N-taurine conjugate. With other bile acids, forms mixed micelles with lecithin in bile which solubilize cholesterol and thus facilitates its excretion. Facilitates fat absorption in the small intestine by micellar solubilization of fatty acids and monoglycerides. Has cathartic properties since it induces fluid secretion from large intestine. Main constituent of the bile of hens, geese and other fowl; occurs in appreciable amounts in the bile of hamster, hog, guinea pig, bear and man. Epimeric with ursodiol,
q.v. Isoln: Windhaus
et al., Z. Physiol. Chem. 140, 177 (1924); Wieland, Reveney,
ibid. 186. Configuration: Lettré,
Ber. 68, 766 (1935). Prepn from cholic acid: Fieser, Rajagopalan,
J. Am. Chem. Soc. 72, 5530 (1950); Hauser
et al., Helv. Chim. Acta 43, 1595 (1960); Hofmann,
Acta Chem. Scand. 17, 173 (1963). Alternate prepns: Sato, Ikekawa,
J. Org. Chem. 24, 1367 (1959); T. Iida, F. C. Chang,
ibid. 46, 2786 (1981). Stereoselective total synthesis: T. Kametani
et al., J. Am. Chem. Soc. 103, 2890 (1981). Asymmetric total synthesis of (+)-form:
eidem, J. Org. Chem. 47, 2331 (1982). Dissolution of cholesterol gallstones: Danzinger
et al., N. Engl. J. Med. 286, 1 (1972); Bell
et al., Lancet II, 1213 (1972). Use in long-term treatment of cerebrotendinous xanthomatosis: V. M. Berginer
et al., N. Engl. J. Med. 311, 1649 (1984). Monograph on bile acids:
The Bile Acids, 2 vols., P. P. Nair, D. Kritchevsky, Eds. (Plenum Press, New York, 1971, 1973). Review of pharmacology and therapeutic use of chenodeoxycholic acid: J. H. Iser, A. Sali,
Drugs 21, 90-119 (1981). Effect on cholesterol and bile acid metabolism: G. S. Tint
et al., Gastroenterology 91, 1007 (1986).
Properties: Needles from ethyl acetate + heptane, mp 119°. [a]D20 +11.5° (dioxane). Freely sol in methanol, alc, acetone, acetic acid; more sol in ether and ethyl acetate than deoxycholic acid. Practically insol in water, petr ether, benzene. High solvent power for alkali soaps, but does not form "choleic" acid addition compds as does deoxycholic acid. Forms beautiful cryst salts of Na, K and Ba. While the acid is tasteless, the Na salt tastes slightly sweet at first, then bitter.
Melting point: mp 119°
Optical Rotation: [a]D20 +11.5° (dioxane)
Derivative Type: Diformate
Molecular Formula: C25H40O6
Molecular Weight: 436.58
Percent Composition: C 68.78%, H 9.23%, O 21.99%
Properties: Clusters of needles from alc; mp with slight effervescence at 137°, upon further heating solidifies again, and finally melts around 172°.
Melting point: mp with slight effervescence at 137°
Derivative Type: Methyl ester
Molecular Formula: C25H42O4
Molecular Weight: 406.60
Percent Composition: C 73.85%, H 10.41%, O 15.74%
Properties: Fine needles from benzene + heptane, mp 90-91°. [a]D25 +20°.
Melting point: mp 90-91°
Optical Rotation: [a]D25 +20°
Therap-Cat: Anticholelithogenic.
Keywords: Cholelitholytic Agent.