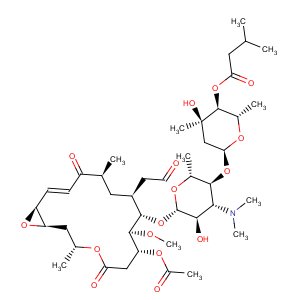
References of Leucomycin V,9-deoxy-12,13-epoxy-12,13-dihydro-9-oxo-, 3-acetate 4B-(3-methylbutanoate),(12S,13S)-
Title: Carbomycin
Literature References: Sixteen-membered-ring macrolide antibiotic complex similar to leucomycin,
q.v. and erythromycin,
q.v., produced by
Streptomyces halstedii. Isoln and antibacterial activity: F. W. Tanner
et al., Antibiot. Chemother. 2, 441 (1952). Two components have been isolated: Carbomycin A (major) and carbomycin B. Isoln of A: Friedman
et al., US 2960438 (1960 to Pfizer); of B: F. A. Hochstein, K. Murai,
J. Am. Chem. Soc. 76, 5080 (1954). Structure of A and B: R. B. Woodward,
Angew. Chem. 69, 50 (1957); revised structure: M. Kuehne, B. W. Benson,
J. Am. Chem. Soc. 87, 4660 (1965); R. B. Woodward
et al., ibid. 4662. Abs config of A and B: W. D. Celmer,
ibid. 88, 5028 (1966). Identity of A with deltamycin A4: Y. Shimauchi
et al., J. Antibiot. 31, 270 (1978). Synthesis of B: K. Tatsuta
et al., J. Am. Chem. Soc. 99, 5826 (1977). Stereospecific total synthesis of B:
eidem, Tetrahedron Lett. 1980, 2837. Retrosynthetic studies: K. C. Nicolaou
et al., J. Am. Chem. Soc. 103, 1222 (1981).
Reviews: D. Vazquez, in
Antibiotics Vol. 1, D. Gottlieb, P. D. Shaw, Eds. (Springer-Verlag, New York, 1967) pp 366-377; W. Keller-Schierlein in
Fortschr. Chem. Org. Naturst. 30, 314-460 (1973).
Derivative Type: Carbomycin A
CAS Registry Number: 4564-87-8
CAS Name: (12
S,13
S)-9-Deoxy-12,13-epoxy-12,13-dihydro-9-oxoleucomycin V 3-acetate 4B-(3-methylbutanoate)
Synonyms: deltamycin A4
Manufacturers' Codes: M-4209
Molecular Formula: C42H67NO16
Molecular Weight: 841.98
Percent Composition: C 59.91%, H 8.02%, N 1.66%, O 30.40%
Properties: Blunt needles from ethanol, mp 214°. [a]D25 -58.6° (chloroform). uv max (abs ethanol): 238, 327 nm (E1%1cm 185, 0.9). Carbomycin standard is the free base having a potency of 1080 units/mg. For stability of soln data
see H. L. Martin,
Antibiot. Chemother. 3, 865 (1953). Weak base, pKb 7.2. Solubilities determined by Weiss
et al., ibid. 7, 374 (1957) in mg/ml at about 28°: water 0.295; methanol >20; ethanol >20. LD50 i.v. in mice: 550 mg/kg (Tanner).
Melting point: mp 214°
pKa: pKb 7.2
Optical Rotation: [a]D25 -58.6° (chloroform)
Absorption maximum: uv max (abs ethanol): 238, 327 nm (E1%1cm 185, 0.9)
Toxicity data: LD50 i.v. in mice: 550 mg/kg (Tanner)
Derivative Type: Carbomycin B
CAS Registry Number: 21238-30-2
CAS Name: 9-Deoxy-9-oxoleucomycin V 3-acetate 4B-(3-methylbutanoate)
Molecular Formula: C42H67NO15
Molecular Weight: 825.98
Percent Composition: C 61.07%, H 8.18%, N 1.70%, O 29.06%
Properties: Colorless anisotropic plates from acetone/water, mp 141-144° (dec), softens at 138°. [a]D25 -35° (c = 1 in chloroform). uv max (abs ethanol): 278 nm (E1%1cm 276). pKb 7.56. Solubilities in mg/ml at 25°: ethanol 450; water 0.1-0.2.
Melting point: mp 141-144° (dec)
pKa: pKb 7.56
Optical Rotation: [a]D25 -35° (c = 1 in chloroform)
Absorption maximum: uv max (abs ethanol): 278 nm (E1%1cm 276)