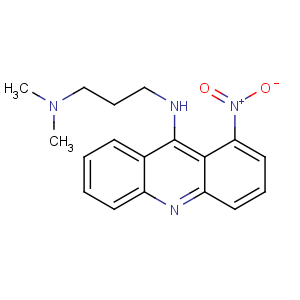
Title: Nitracrine
CAS Registry Number: 4533-39-5
CAS Name: N,N-Dimethyl-
N¢-(1-nitro-9-acridinyl)-1,3-propanediamine
Synonyms: 9-[[3-(dimethylamino)propyl]amino]-1-nitroacridine
Molecular Formula: C18H20N4O2
Molecular Weight: 324.38
Percent Composition: C 66.65%, H 6.21%, N 17.27%, O 9.86%
Literature References: Deriv of acridine,
q.v., with cytostatic and cytotoxic properties. Prepn:
FR 1458183 (1966 to Polfa),
C.A. 68, 39493s (1968); A. Ledochowski, B. Stefanska,
Rocz. Chem. 40, 301 (1966),
C.A. 65, 2219b (1966). Pharmacological studies: J. Gieldanowski
et al., Arch. Immunol. Ther. Exp. 20, 399 (1972);
eidem, ibid. 419. Mechanism of action: J. Konopa
et al., Mater. Med. Pol. 8, 258 (1976). Cytotoxicity study: I. Szumiel, M. Walicka,
Neoplasma 27, 697 (1980). DNA binding activity: L. Szmigiero, M. Gniazdowski,
Arzneim.-Forsch. 31, 1875 (1981). Comprehensive review: M. Gniazdowski
et al. in
Antibiotics Vol. V, part 2, F. E. Hahn, Ed. (Springer-Verlag, New York, 1979) pp 275-297.
Properties: Crystals from benzene/petr ether, mp 134-135°. Practically insol in water. Sol in most organic solvents. pKa1 6.45; pKa2 8.8.
Melting point: mp 134-135°
pKa: pKa1 6.45; pKa2 8.8
Derivative Type: Dihydrochloride monohydrate
CAS Registry Number: 55429-45-3
Manufacturers' Codes: C-283
Trademarks: Ledakrin (Polfa)
Molecular Formula: C18H20N4O2.2HCl.H2O
Molecular Weight: 415.31
Percent Composition: C 52.06%, H 5.82%, N 13.49%, O 11.56%, Cl 17.07%
Properties: Orange crystals, mp 223-224°. Sol in water, methanol, ethanol, slightly sol in benzene, diethyl ether. Conc water solns are acidic (pH 4). LD50 in rats, mice (mg/kg): 1, 0.72 i.v.; 34, 26 i.g. (Gniazdowski).
Melting point: mp 223-224°
Toxicity data: LD50 in rats, mice (mg/kg): 1, 0.72 i.v.; 34, 26 i.g. (Gniazdowski)
Therap-Cat: Antineoplastic.
Keywords: Antineoplastic.