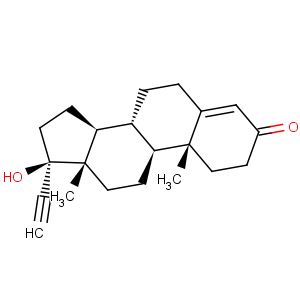
Title: Ethisterone
CAS Registry Number: 434-03-7
CAS Name: 17a-Hydroxypregn-4-en-20-yn-3-one
Synonyms: 17a-ethynyltestosterone; 17a-ethynyl-17b-hydroxy-4-androsten-3-one; 17a-ethinyltestosterone; 17a-ethynyl-4-androsten-17b-ol-3-one; anhydrohydroxyprogesterone; pregneninolone
Molecular Formula: C21H28O2
Molecular Weight: 312.45
Percent Composition: C 80.72%, H 9.03%, O 10.24%
Literature References: Synthetic progestogen; metabolite of danazol,
q.v.; intermediate in the synthesis of spironolactone,
q.v. Prepn: Inhoffen
et al., Ber. 71, 1024 (1938). Crystal structure and photostability study: J. Reisch
et al., Monatsh. Chem. 124, 1169 (1993). GC/MS characterization as a metabolite of danazol: J. Y. Kim
et al., J. Vet. Pharmacol. Ther. 24, 147 (2001). HPLC/CD determn of isomers: D. Szegvári
et al., Anal. Bioanal. Chem. 375, 713 (2003).
Properties: Crystals from ethyl acetate, mp 269-275°. Sublimes in high vacuum at 190-195°. [a]D23 +23.8° (dioxane); [a]D25 -32.0° (pyridine). Crystal density: 1.220 g/cm3. uv max (methanol): 241 nm (E1%1cm 513). Practically insol in water. Slightly sol in alcohol, acetone, ether, chloroform, vegetable oils.
Melting point: mp 269-275°
Optical Rotation: [a]D23 +23.8° (dioxane); [a]D25 -32.0° (pyridine)
Absorption maximum: uv max (methanol): 241 nm (E1%1cm 513)