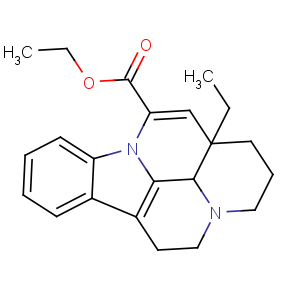
Title: Vinpocetine
CAS Registry Number: 42971-09-5
CAS Name: (3a,16a)-Eburnamenine-14-carboxylic acid ethyl ester
Synonyms: 3a,16a-apovincaminic acid ethyl ester; ethyl apovincamin-22-oate
Manufacturers' Codes: RGH-4405
Trademarks: Cavinton (Thiemann)
Molecular Formula: C22H26N2O2
Molecular Weight: 350.45
Percent Composition: C 75.40%, H 7.48%, N 7.99%, O 9.13%
Literature References: Deriv of vincamine,
q.v., with vasodilating activity. Prepn: C. L?rincz
et al., DE 2253750;
eidem, US 4035370 (1973, 1977 both to Gedeon Richter);
eidem, Arzneim.-Forsch. 26, 1907 (1976). Series of articles on pharmacology, biochemistry, metabolism, pharmacokinetics, clinical studies:
ibid. 1908-1989. Toxicity studies: E. Pálosi, L. Szporny,
ibid. 1926; E. Cholnoky, L. I. D?m?k,
ibid. 1939. HPLC studies: G. Szepesi, M. Gazdag,
J. Chromatogr. 205, 57, 341 (1981). Evaluation of effectiveness as antimotion drug: E. I. Matsnev, D. Bodo,
Aviat. Space Environ. Med. 55, 281 (1984). One-pot synthesis from vincamine: Y. Kuge
et al., Synth. Commun. 24, 759 (1994).
Properties: Crystals from benzene, mp 147-153° (dec). [a]D20 +114° (c = 1 in pyridine). uv max (96% ethanol): 229, 275, 315 nm (log e 4.45, 4.08, 3.85). LD50 in mice, rats (mg/kg): 534, 503 orally; 240, 133.8 i.p.; 58.7, 42.6 i.v. (Pálosi, Szporny), also reported as 161.2 mg/kg i.p. in mice (Cholnoky, D?m?k).
Melting point: mp 147-153° (dec)
Optical Rotation: [a]D20 +114° (c = 1 in pyridine)
Absorption maximum: uv max (96% ethanol): 229, 275, 315 nm (log e 4.45, 4.08, 3.85)
Toxicity data: LD50 in mice, rats (mg/kg): 534, 503 orally; 240, 133.8 i.p.; 58.7, 42.6 i.v. (Pálosi, Szporny), also reported as 161.2 mg/kg i.p. in mice (Cholnoky, D?m?k)
Therap-Cat: Vasodilator (cerebral).
Keywords: Vasodilator (Cerebral).