References of Cyclopentanecarboxamide,3-amino-N-(3-amino-3-iminopropyl)-, (1R,3S)-
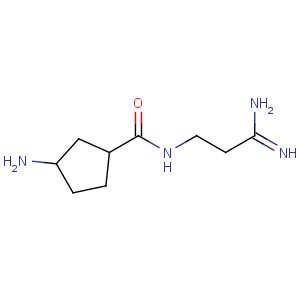
Title: Amidinomycin
CAS Registry Number: 3572-60-9
CAS Name: 3-Amino-
N-(3-amino-3-iminopropyl)cyclopentanecarboxamide
Synonyms: N-(2¢-amidinoethyl)-3-aminocyclopentanecarboxamide; myxoviromycin
Molecular Formula: C9H18N4O
Molecular Weight: 198.27
Percent Composition: C 54.52%, H 9.15%, N 28.26%, O 8.07%
Literature References: Antibiotic substance produced by
Streptomyces flavochromogenes isolated from Japanese soil (Shiuoka Prefecture). Isoln and structure: S. Nakamura
et al., J. Antibiot. 14A, 103 (1961); S. Nakamura,
Chem. Pharm. Bull. 9, 641 (1961). Identity with myxoviromycin: S. Nakamura
et al., J. Antibiot. 14A, 163 (1961). Prepn: Katsube, Saito,
JP 68 21418 (1968 to Sumitomo),
C.A. 70, 87135q (1969). Synthesis of amidinomycin and
trans isomer: H. Paul
et al., Arch. Pharm. 301, 512 (1968). Crystal and molecular structure: M. Kaneda
et al., J. Antibiot. 33, 778 (1980).
Derivative Type: Sulfate
Molecular Formula: C9H18N4O.H2SO4
Molecular Weight: 296.34
Percent Composition: C 36.48%, H 6.80%, N 18.91%, O 27.00%, S 10.82%
Properties: Plates or needles from water + methanol, dec 285-288°. [a]D21 -3.9° (c = 3). Absorption spectra: S. Nakamura,
loc. cit. Soluble in water. Practically insol in ether, benzene, ethyl acetate, methanol, ethanol, butanol, acetone.
Optical Rotation: [a]D21 -3.9° (c = 3)
Therap-Cat: Antiviral.
Keywords: Antiviral.