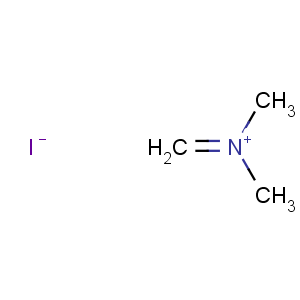
Title: Dimethyl(methylene)ammonium Iodide
CAS Registry Number: 33797-51-2
CAS Name: N-Methyl-
N-methylenemethanaminium iodide
Synonyms: Eschenmoser's salt
Molecular Formula: C3H8IN
Molecular Weight: 185.01
Percent Composition: C 19.48%, H 4.36%, I 68.59%, N 7.57%
Line Formula: H2C=N(CH3)2I
Literature References: Mannich type intermediate originally developed to introduce methyl groups into corrin chromophore. Prepn from trimethylamine and diiodomethane: J. Schreiber
et al., Angew. Chem. Int. Ed. 10, 330 (1971); from
N,N,N¢,N¢-tetramethylmethylenediamine: T. A. Bryson
et al., J. Org. Chem. 45, 524 (1980). Used in prepn of Mannich bases: J. Hooz, J. N. Bridson,
J. Am. Chem. Soc. 95, 602 (1973). In functionalization of indoles: A. P. Kozikowski, H. Isida,
Heterocycles 14, 55 (1980). In prepn of a-methylene carbonyls: J. L. Roberts
et al., Tetrahedron Lett. 1977, 1621; of terminal olefins:
eidem, ibid. 1299.
Properties: Colorless crystals from tetrahydrothiophene dioxide, dec ~240°. Sublimes at 120° at 0.05 torr.
Use: In organic synthesis.