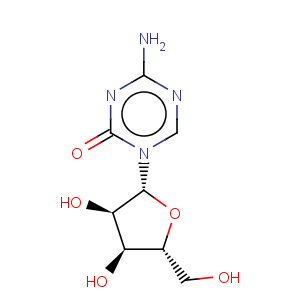
Title: Azacitidine
CAS Registry Number: 320-67-2
CAS Name: 4-Amino-1-b-D-ribofuranosyl-1,3,5-triazin-2(1
H)-one
Synonyms: 5-azacytidine; 5-AzaC; ladakamycin
Manufacturers' Codes: U-18496; NSC-102816
Trademarks: Mylosar (formerly); Vidaza (Pharmion)
Molecular Formula: C8H12N4O5
Molecular Weight: 244.20
Percent Composition: C 39.35%, H 4.95%, N 22.94%, O 32.76%
Literature References: DNA methylation inhibitor; analog of the pyrimidine nucleoside, cytidine,
q.v. Chemical synthesis: A. Piskala, F. Sorm,
Collect. Czech. Chem. Commun. 29, 2060 (1964); M. W. Winkley, R. K. Robins,
J. Org. Chem. 35, 491 (1970). Production by fermentation of
Streptoverticillium ladakanus and activity: L. J. Hanka
et al., Antimicrob. Agents Chemother. 1966, 619; M. E. Bergy, R. R. Herr,
ibid. 625. HPLC determn in pharmaceutical prepns: L. D. Kissinger, N. L. Stemm,
J. Chromatogr. 353, 309 (1986). Toxicology study: P. E. Palm, C. J. Kensler,
U.S. Clearinghouse Fed. Sci. Tech. Inform. (PB-194791, 1970) 191 pp.,
C.A. 75, 33704j (1971). Review of clinical experience in acute nonlymphocytic leukemia: A. B. Glover
et al., Cancer Treat. Rep. 71, 737-746 (1987); of mechanism of action: A. B. Glover, B. Leyland-Jones,
ibid. 959-964. Review of carcinogenic risk:
IARC Monographs 50, 47-63 (1990). Clinical efficacy in b-thalassemia: C. H. Lowrey, A. W. Nienhuis,
N. Engl. J. Med. 329, 845 (1993); in myelodysplastic syndrome: L. R. Silverman
et al., J. Clin. Oncol. 20, 2429 (2002); A. B. Kornblith
et al., ibid. 2441.
Properties: Crystals from aq ethanol, mp 235-237° (dec). [a]D26 +22.4° (c = 1 in water). uv max (water): 241 nm (e 8767); (0.01
N HCl): 249 nm (e 3077); (0.01
N KOH): 223 nm (e 24200). Soly (mg/ml): 40 warm water, 14 cold water, 28 0.1
N HCl, 43 0.1
N NaOH, 52.7 DMSO, 1 acetone, 1 chloroform, 1 hexane. LD50 in mice (mg/kg): 115.9 i.p.; 572.3 orally (Palm, Kensler).
Melting point: mp 235-237° (dec)
Optical Rotation: [a]D26 +22.4° (c = 1 in water)
Absorption maximum: uv max (water): 241 nm (e 8767); (0.01
N HCl): 249 nm (e 3077); (0.01
N KOH): 223 nm (e 24200)
Toxicity data: LD50 in mice (mg/kg): 115.9 i.p.; 572.3 orally (Palm, Kensler)
CAUTION: This substance is reasonably anticipated to be a human carcinogen:
Report on Carcinogens, Eleventh Edition (PB2005-104914, 2004) p III-24.
Therap-Cat: Antineoplastic.
Keywords: Antineoplastic; Antimetabolites; Pyrimidine Analogs.