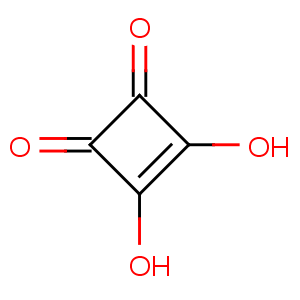
Title: Squaric Acid
CAS Registry Number: 2892-51-5
CAS Name: 3,4-Dihydroxy-3-cyclobutene-1,2-dione
Synonyms: 1,2-dihydroxycyclobutenedione; diketocyclobutenediol
Molecular Formula: C4H2O4
Molecular Weight: 114.06
Percent Composition: C 42.12%, H 1.77%, O 56.11%
Literature References: Dibasic acid; two-dimensional molecular antiferroelectric material. Prepn: S. Cohen
et al., J. Am. Chem. Soc. 81, 3480 (1959); J. D. Park
et al., ibid. 84, 2919 (1962). Aqueous dissociation study: L. M. Schwartz, L. O. Howard,
J. Phys. Chem. 74, 4374 (1970). Structure determn studies: D. Semmingsen,
Acta Chem. Scand. 27, 3961 (1973); Y. Wang
et al., J. Chem. Soc. Perkin Trans. 2 1974, 35. Review of syntheses and reactivity: G. Maahs, P. Hegenberg,
Angew. Chem. Int. Ed. 5, 888-893 (1966); of coordination chemistry: L. A. Hall, D. J. Williams,
Adv. Inorg. Chem. 52, 249-291 (2001).
Properties: Crystals from water, dec 293°. d 1.90. uv max (H2O): 269.5 nm (e 37000). Soly: 7% boiling water; 2% room temp water. Insol in acetone, ether. pK1 ~0.6 (25°). pK2 3.480 ±0.023 (25°).
Melting point: dec 293°
pKa: pK1 ~0.6; pK2 3.480 ±0.023 (25°).
Absorption maximum: uv max (H2O): 269.5 nm (e 37000)
Density: d 1.90
Derivative Type: Dibutylester
CAS Registry Number: 2892-62-8
CAS Name: 3,4-Dibutoxy-3-cyclobutene-1,2-dione
Synonyms: dibutyl squarate
Molecular Formula: C12H18O4
Molecular Weight: 226.27
Percent Composition: C 63.70%, H 8.02%, O 28.28%
Literature References: Prepn: G. Maahs,
Ann. 686, 55 (1965). Clinical evaluation in alopecia: G. Micali
et al., Int. J. Dermatol. 35, 52 (1996); in treatment of warts:
idem et al., Pediatr. Dermatol. 17, 315 (2000).
Properties: bp0.5 138-139°.
nD20 1.4943.
Boiling point: bp0.5 138-139°
Index of refraction: nD20 1.4943
Use: Squaric acid and its derivatives are used in synthesis of pharmaceutical intermediates, squarylium dyes, and photoconducting squaraines.
Therap-Cat: Dibutylester as contact sensitizer for treatment of alopecia and warts.