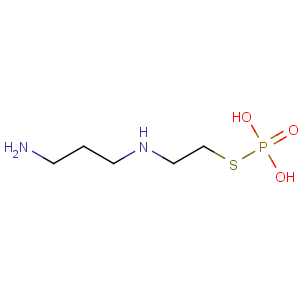
Title: Amifostine
CAS Registry Number: 20537-88-6
CAS Name: 2-[(3-Aminopropyl)amino]ethanethiol dihydrogen phosphate (ester)
Synonyms: phosphorothioic acid
S-[2-[(3-aminopropyl)amino]ethyl] ester; aminopropylaminoethyl thiophosphate; ethiofos; gammaphos; SAPEP
Manufacturers' Codes: NSC-296961; WR-2721; YM-08310
Trademarks: Ethyol (U.S. Bioscience)
Molecular Formula: C5H15N2O3PS
Molecular Weight: 214.22
Percent Composition: C 28.03%, H 7.06%, N 13.08%, O 22.41%, P 14.46%, S 14.97%
Literature References: Thiophosphate derivative of cysteamine,
q.v.; provides normal cells with selective protection against the toxic effects of cancer chemotherapy and radiation treatment. Prepn of monohydrate: J. R. Piper
et al., J. Med. Chem. 12, 236 (1969); J. R. Piper, T. P. Johnston,
US 3892824 (1975 to Southern Res. Inst.). Differential radioprotective activity: J. M. Yuhas, J. B. Storer,
J. Natl. Cancer Inst. 42, 331 (1969). Mechanism of action study: G. D. Smoluk
et al., Cancer Res. 48, 3641 (1988). Bioavailability: L. Fleckenstein
et al., Pharmacol. Ther. 39, 203 (1988). Clinical pharmacokinetics: L. M. Shaw
et al., ibid. 195. HPLC determn in plasma: N. F. Swynnerton
et al., Int. J. Radiat. Oncol. Biol. Phys. 12, 1495 (1986). Review of development as radioprotector: D. Q. Brown
et al., Pharmacol. Ther. 39, 157-168 (1988); of role in chemotherapy: R. L. Capizzi
et al., Cancer 72, 3495-3501 (1993); M. Treskes, W. J. M. van der Vijgh,
Cancer Chemother. Pharmacol. 33, 93-106 (1993).
Derivative Type: Monohydrate
CAS Registry Number: 63717-27-1
Properties: White solid from methanol/ether, mp 160-161° (dec). LD50 in mice (mg/kg): 700 i.p. (Piper, Johnston).
Melting point: mp 160-161° (dec)
Toxicity data: LD50 in mice (mg/kg): 700 i.p. (Piper, Johnston)
Derivative Type: Trihydrate
CAS Registry Number: 112901-68-5
Properties: Soly in water: >9 g/100 ml. pKa1 <2.0; pKa2 4.2; pKa3 9.0; pKa4 11.7.
pKa: pKa1 <2.0; pKa2 4.2; pKa3 9.0; pKa4 11.7
Therap-Cat: Radioprotective agent.
Keywords: Antineoplastic Adjunct; Radioprotective.