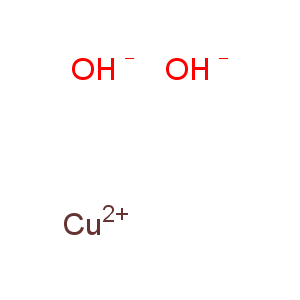
Title: Cupric Hydroxide
CAS Registry Number: 20427-59-2
Synonyms: Copper hydrate; hydrated cupric oxide
Molecular Formula: CuH2O2
Molecular Weight: 97.56
Percent Composition: Cu 65.14%, H 2.07%, O 32.80%
Line Formula: Cu(OH)2
Literature References: Commercial prepn: Furness,
US 1800828 (1931 to Cellosilk);
idem, US RE 24324 (1957 to Copper Research); Rowe,
US 2536096 (1951 to Mountain Copper); laboratory prepn: Weiser
et al., J. Am. Chem. Soc. 64, 503 (1942); Gauthier,
Bull. Soc. Chim. Fr. 1960, 353.
Properties: Blue to blue-green gel or light blue cryst powder. Stability is dependent on the method of prepn; may dec to black CuO on standing a few days or on heating. d 3.37. Practically insol in water. Sol in concd alkali when freshly pptd; sol in acids, NH4OH.
Density: d 3.37
Use: In manuf of rayon, battery electrodes, other Cu salts; as mordant in dyeing; as pigment; in fungicides, insecticides; as feed additive; in treating and staining paper; in prepn of Schweitzer's reagent; in catalysts.