Title: Ferric Hydroxide
CAS Registry Number: 20344-49-4
CAS Name: Ferric hydroxide oxide
Synonyms: hydrated ferric oxide
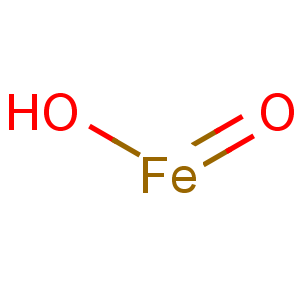
Molecular Formula: FeHO2
Molecular Weight: 88.85
Percent Composition: Fe 62.85%, H 1.13%, O 36.01%
Line Formula: FeO(OH)
Literature References: Occurs in nature as the minerals
goethite [a-FeO(OH)],
lepidocrocite [g-FeO(OH)], and
limonite [FeO(OH).
nH2O]. Other known allomorphic forms: b-FeO(OH); d-FeO(OH). The hydroxide Fe(OH)3 is not known. Prepn: Lux in
Handbook of Preparative Inorganic Chemistry vol 2, G. Brauer, Ed. (Academic Press, New York, 2nd ed., 1965) p 1499. Crystal structure of a-FeO(OH): Sampson,
Acta Crystallogr. 25B, 1683 (1969).
Review: Bernal
et al., Clay Miner. Bull. 4, 15-30 (1959).
Properties: Red to brown powder or crystals. Loses H2O to form Fe2O3. d 3.4-3.9. Practically insol in water, alcohol. Sol in mineral acids.
Density: d 3.4-3.9
Use: In purifying water; as absorbent in chemical processing; as pigment; as catalyst.