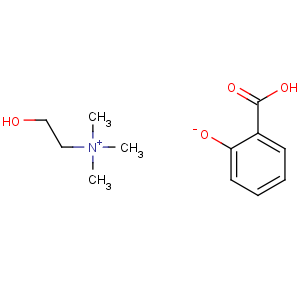
Title: Choline Salicylate
CAS Registry Number: 2016-36-6
CAS Name: 2-Hydroxy-
N,N,N-trimethylethanaminium salt with 2-hydroxybenzoic acid (1:1)
Synonyms: (2-hydroxyethyl)trimethylammonium salicylate; choline salicylic acid salt; salicylic acid choline salt
Trademarks: Actasal (Purdue Frederick); Arthropan (Purdue Frederick); Artrobione; Audax (Napp); Mundisal (Purdue Frederick)
Molecular Formula: C12H19NO4
Molecular Weight: 241.28
Percent Composition: C 59.73%, H 7.94%, N 5.81%, O 26.52%
Literature References: Prepn from choline chloride and sodium salicylate: Broh-Kahn,
Int. Rec. Med. 173, 219 (Apr. 1960);
cf. Johnson,
GB 8031 (1919); Broh-Kahn
et al., US 3069321 (1962 to Labs. for Pharmaceut. Dev.). Variations of process:
BE 583513 (1960 to Mundipharma, AG).
Properties: Extremely hygroscopic solid, mp 49.5-50.0°. Very freely sol in water. Also sol in alcohol, acetone, other hydrophilic solvents. Practically insol in ether, petr ether, benzene, oils. Aq solns are stable, they contain the compd in the form of its dissociated choline and salicylate ions. pH of 10% aq soln 6.5. Aq solns are easily discolored by minute traces of iron. The addition of acid to aq solns immediately precipitates free salicylic acid, while choline base, readily recognized by its fishy odor, is liberated upon the addition of alkali.
Melting point: mp 49.5-50.0°
Therap-Cat: Analgesic; antipyretic.
Keywords: Analgesic (Non-Narcotic); Antipyretic.