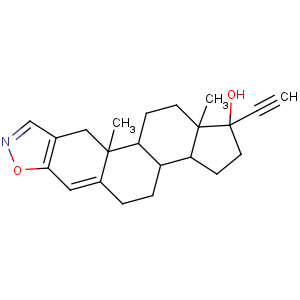
Title: Danazol
CAS Registry Number: 17230-88-5
CAS Name: (17a)-Pregna-2,4-dien-20-yno[2,3-
d]isoxazol-17-ol
Synonyms: 1-ethynyl-2,3,3a,3b,4,5,10,10a,10b,11,12,12a-dodecahydro-10a,12a-dimethyl-1
H-cyclopenta[7,8]phenanthro[3,2-
d]isoxazol-1-ol; 17a-ethynyl-17b-hydroxy-4-androsteno[2,3-
d]isoxazole
Manufacturers' Codes: Win-17757
Trademarks: Bonzol (Tokyo Tanabe); Cyclomen (Sanofi-Synthelabo); Danatrol (Sanofi-Synthelabo); Danocrine (Sanofi-Synthelabo); Danol (Sanofi-Synthelabo); Danoval (Krka); Ladogal (Sanofi-Synthelabo); Winobanin (Sanofi-Synthelabo)
Molecular Formula: C22H27NO2
Molecular Weight: 337.46
Percent Composition: C 78.30%, H 8.06%, N 4.15%, O 9.48%
Literature References: Anterior pituitary supressant. Anabolic steroid deriv of ethisterone,
q.v., with mild androgenic side effects (an impeded androgen). Prepn:
GB 905844 (1962 to Sterling Drug),
C.A. 58, 6895c (1963); Manson
et al., J. Med. Chem. 6, 1 (1963); Clinton, Manson,
US 3135743 (1964 to Sterling Drug). Activity studies: Sherins
et al., J. Clin. Endocrinol. Metab. 32, 522 (1971); Dmowski
et al., Fertil. Steril. 22, 9 (1971). Clinical studies in endometriosis and other endocrine disorders: R. B. Greenblatt
et al., ibid. 102. Series of articles on pharmacology, pharmacokinetics and clinical use:
Drugs 19, 321-372 (1980). Use in idiopathic thrombocytopenic purpura: Y. S. Ahn
et al., N. Engl. J. Med. 308, 1396 (1983); in hemophilia: H. R. Gralnick
et al., ibid. 1393.
Properties: Crystals from acetone, mp 224.4-226.8°. [a]D25 +7.5° (ethanol); [a]D25 +21.9° (chloroform). uv max (ethanol): 286 nm (e 11300).
Melting point: mp 224.4-226.8°
Optical Rotation: [a]D25 +7.5° (ethanol); [a]D25 +21.9° (chloroform)
Absorption maximum: uv max (ethanol): 286 nm (e 11300)
Therap-Cat: Antigonadotropin.
Keywords: Antigonadotropin.