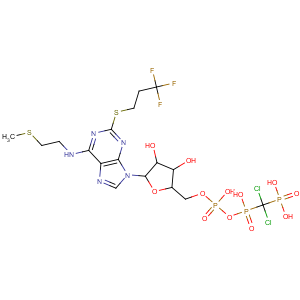
Title: Cangrelor
CAS Registry Number: 163706-06-7
CAS Name: N-[2-(Methylthio)ethyl]-2-[(3,3,3-trifluoropropyl)thio]-5¢-adenylic acid monoanhydride with (dichloromethylene)bis[phosphonic acid]
Synonyms: N6-(2-methylthioethyl)-2-(3,3,3-trifluoropropylthio)-b,g-dichloromethylene ATP
Manufacturers' Codes: AR-C69931XX
Molecular Formula: C17H25Cl2F3N5O12P3S2
Molecular Weight: 776.36
Percent Composition: C 26.30%, H 3.25%, Cl 9.13%, F 7.34%, N 9.02%, O 24.73%, P 11.97%, S 8.26%
Literature References: Specific P2Y12 purinoceptor antagonist; inhibits ADP-induced platelet aggregation. Prepn: A. H. Ingall
et al., WO 9418216 (1994 to Fisons);
eidem,
US 5721219 (1998 to Astra); and
in vivo antithrombotic activity:
idem et al., J. Med. Chem. 42, 213 (1999).
In vivo antithrombotic effects in canine arterial thrombosis: J. Huang
et al., J. Pharmacol. Exp. Ther. 295, 492 (2000). Mechanism of action study: A. Ishii-Watabe
et al., Biochem. Pharmacol. 59, 1345 (2000). Clinical safety assessment and evaluation in acute coronary syndromes: R. F. Storey
et al., Thromb. Haemostasis 85, 401 (2001); in angina pectoris and non-Q-wave myocardial infarction: F. Jacobsson
et al., Clin. Ther. 24, 752 (2002). Clinical pharmacodynamics compared with clopidogrel: R. F. Storey
et al., Platelets 13, 407 (2002). Review of clinical development: S. C. Chattaraj,
Curr. Opin. Invest. Drugs 2, 250-255 (2001).
Derivative Type: Tetrasodium salt
CAS Registry Number: 163706-36-3
Manufacturers' Codes: AR-C69931MX
Molecular Formula: C17H21Cl2F3N5Na4O12P3S2
Molecular Weight: 864.29
Percent Composition: C 23.62%, H 2.45%, Cl 8.20%, F 6.59%, N 8.10%, Na 10.64%, O 22.21%, P 10.75%, S 7.42%
Properties: Freely sol in water.
Therap-Cat: Antithrombotic.
Keywords: Antithrombotic.