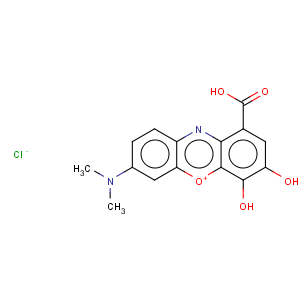
Title: Gallocyanine
CAS Registry Number: 1562-85-2
CAS Name: 1-Carboxy-7-(dimethylamino)-3,4-dihydroxyphenoxazin-5-ium chloride
Synonyms: C.I. Mordant Blue 10; C.I. 51030
Molecular Formula: C15H13ClN2O5
Molecular Weight: 336.73
Percent Composition: C 53.50%, H 3.89%, Cl 10.53%, N 8.32%, O 23.76%
Literature References: Made by introducing nitrosodimethylaniline hydrochloride into a suspension of gallic acid in boiling methanol: Koechlin,
DE 19580 (1881),
Frdl. 1, 269; Nietzki, Otto,
Ber. 21, 1740 (1888);
Colour Index vol. 4 (3rd ed., 1971) p 4460.
Properties: Green crystals. Practically insol in cold water. Slightly sol in hot water; sol in alcohol, glacial acetic acid, in alkali carbonates with reddish color, in concd HCl with a blue color which becomes red upon diluting with water.
Use: As a dye; in alkali carbonate soln as a reagent for lead with which it forms a deep violet color.