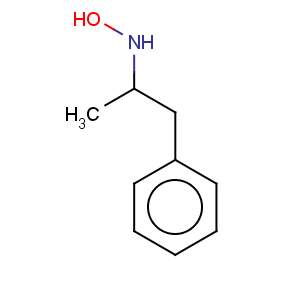
Title: Hydroxyamphetamine
CAS Registry Number: 1518-86-1
CAS Name: 4-(2-Aminopropyl)phenol
Synonyms: dl-p-hydroxy-a-methylphenethylamine;
dl-1-
p-hydroxyphenyl-2-propylamine;
p-hydroxyphenylisopropylamine; a-methyltyramine
Trademarks: Paredrine (SK & F); Paredrinex (SK & F); Pulsoton
Molecular Formula: C9H13NO
Molecular Weight: 151.21
Percent Composition: C 71.49%, H 8.67%, N 9.26%, O 10.58%
Literature References: Prepn from oxime of
p-methoxyphenyl acetone: Mannich, Jacobsohn,
Ber. 43, 189 (1910),
DE 243546. From
p-nitrobenzyl chloride and a salt of nitroethane: Hoover, Hass,
J. Org. Chem. 12, 501 (1947).
Properties: Crystals (rosettes) from benzene, mp 125-126°. Sol in water, alcohol, chloroform, ethyl acetate.
Melting point: mp 125-126°
Derivative Type: Iodide
Molecular Formula: C9H14INO
Molecular Weight: 279.12
Percent Composition: C 38.73%, H 5.06%, I 45.47%, N 5.02%, O 5.73%
Properties: Stout prisms, mp 155°. Freely sol in water, alcohol, acetone.
Melting point: mp 155°
Derivative Type: Hydrochloride
Molecular Formula: C9H14ClNO
Molecular Weight: 187.67
Percent Composition: C 57.60%, H 7.52%, Cl 18.89%, N 7.46%, O 8.53%
Properties: Crystals from HCl, mp 171-172°. Sol in water, alcohol. Practically insol in ether.
Melting point: mp 171-172°
Derivative Type: Hydrobromide
CAS Registry Number: 306-21-8
Molecular Formula: C9H14BrNO
Molecular Weight: 232.12
Percent Composition: C 46.57%, H 6.08%, Br 34.42%, N 6.03%, O 6.89%
Properties: Crystals. Freely sol in water, alcohol, acetone. Prepd as Paredrine Hydrobromide Aqueous?a 1% aq soln made isotonic with sodium chloride and preserved with sodium ethylmercuri thiosalicylate. Also prepd as Paredrine Hydrobromide Ophthalmic 1%, with boric acid?a 1% aq soln made tear-isotonic with 2% boric acid and preserved with sodium ethylmercuri thiosalicylate.
Therap-Cat: Adrenergic (ophthalmic); mydriatic.
Keywords: a-Adrenergic Agonist; Mydriatic.