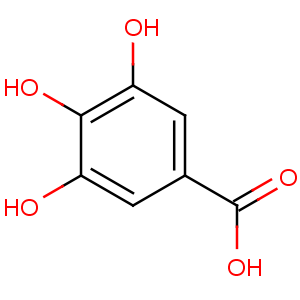
Title: Gallic Acid
CAS Registry Number: 149-91-7
CAS Name: 3,4,5-Trihydroxybenzoic acid
Molecular Formula: C7H6O5
Molecular Weight: 170.12
Percent Composition: C 49.42%, H 3.55%, O 47.02%
Literature References: Obtained by alkaline or acid hydrolysis of the tannins from nutgalls; also by enzymatic hydrolysis using spent broths from
Penicillium glaucum or
Aspergillus niger which contain tannase: A. G. Perkin, O. Gunnell,
J. Chem. Soc. 69, 1303 (1896); Hsias,
Huang Hai No. 7, 51 (1946),
C.A. 42, 3901i (1948); Cochrane,
Econ. Bot. 2, 145 (1948); Toth, Henster,
Acta Chim. Acad. Sci. Hung. 2, 209 (1952). Prepn from tannin containing materials: Krueger
et al., US 2723992 (1955 to Mallinckrodt). Synthesis from aliphatic materials: Shipchandler
et al., J. Chem. Soc. Perkin Trans. 1 1975, 1400. Biosynthesis: Haslam
et al., J. Chem. Soc. 1961, 1854. Study of polymorphic forms: E. Lindpainter,
Mikrochemie 27, 21 (1939). Toxicity studies: J. W. Dollahite
et al., Am. J. Vet. Res. 23, 1264 (1962).
Properties: Needles from abs methanol or chloroform, formerly reported as dec 235-240° (Perkin, Gunnell). Sublimes at 210° giving a stable form with mp 258-265° (dec) and an unstable form mp 225-230° (Lindpainter). One gram dissolves in 87 ml water, 3 ml boiling water, 6 ml alcohol, 100 ml ether, 10 ml glycerol, 5 ml acetone. Practically insol in benzene, chloroform, petr ether.
Protect from light. LD50 in rabbits (g/kg): 5.0 orally (Dollahite).
Melting point: mp 258-265° (dec) and an unstable form mp 225-230° (Lindpainter)
Toxicity data: LD50 in rabbits (g/kg): 5.0 orally (Dollahite)
Derivative Type: Methyl ester
CAS Registry Number: 99-24-1
Synonyms: Methyl gallate; gallicin
Molecular Formula: C8H8O5
Molecular Weight: 184.15
Percent Composition: C 52.18%, H 4.38%, O 43.44%
Properties: Monoclinic prisms from methanol, often hydrated or solvated. When dry, mp 202°. Sol in hot water, alcohol, methanol, ether.
Melting point: mp 202°
Derivative Type: Propyl ester
see Propyl Gallate
Use: Manuf gallic acid esters, pyrogallol, inks; as photographic developer; in tanning; in dyeing; in testing for free mineral acids, dihydroxyacetone and alkaloids. Esters as antioxidants.
Therap-Cat: Formerly as astringent, styptic.
Therap-Cat-Vet: Has been used as intestinal astringent.
Keywords: Astringent.