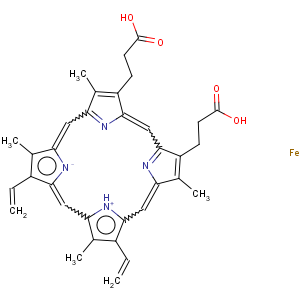
Title: Heme
CAS Registry Number: 14875-96-8
CAS Name: (
SP-4-2)-[7,12-Diethenyl-3,8,13,17-tetramethyl-21
H,23
H-porphine-2,18-dipropanoato(4-)-
N21
,N22
,N23
,N24]ferrate(2-) dihydrogen
Synonyms: [dihydrogen 3,7,12,17-tetramethyl-8,13-divinyl-2,18-porphinedipropionato(2-)]iron; 1,3,5,8-tetramethyl-2,4-divinylporphine-6,7-dipropionic acid ferrous complex; ferroheme; hem; protoheme; protoheme IX; reduced hematin; ferroprotoporphyrin
Molecular Formula: C34H32FeN4O4
Molecular Weight: 616.49
Percent Composition: C 66.24%, H 5.23%, Fe 9.06%, N 9.09%, O 10.38%
Literature References: Heme occurs free in tissues in the presence of certain pathological conditions, and in normal tissues; it occurs as the prosthetic group of a number of hemoproteins. It has been identified as the prosthetic group of hemoglobins, erythrocruorins (the hemoglobin analog of many invertebrates), myoglobins, some peroxidases, catalases, and cytochromes b. It is the color-furnishing portion of hemoglobin. Obtained when a soln of hematin in alkali is reduced in absence of nitrogenous substances: Bertin-Sans, de Moitessier,
Compt. Rend. 114, 923 (1892); Dhéré
et al., ibid. 165, 515 (1917). Synthesis: Fischer-Orth,
Die Chemie des Pyrrols II, 1, 384 (Leipzig, 1937). Biosynthesis: Shemin,
Naturwissenschaften 57, 185 (1970).
Review: J. E. Falk,
Porphyrins and Metalloporphyrins (Elsevier, NewYork, 1964) pp 8, 94, 183. Comprehensive monograph: Chance
et al., Hemes and Hemoproteins (Academic Press, New York, 1966) 624 pp;
Handbook of Experimental Pharmacology vol. 44, entitled "Heme and Hemoproteins", F. DeMatteis, W. N. Aldridge, Eds. (Springer-Verlag, New York, 1978) 449 pp.
Properties: Fine brown needles with a dark violet sheen. Absorption max in phosphate buffer at pH 7: ~550, 575 nm (E572mM 5.5). Sparingly sol in glacial acetic acid; freely sol in the presence of oxygen. Very unstable.
Absorption maximum: Absorption max in phosphate buffer at pH 7: ~550, 575 nm (E572mM 5.5)