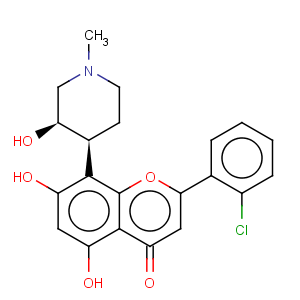
Title: Flavopiridol
CAS Registry Number: 146426-40-6
CAS Name: rel-(-)-2-(2-Chlorophenyl)-5,7-dihydroxy-8-[(3
R,4
S)-3-hydroxy-1-methyl-4-piperidinyl]-4
H-1-benzopyran-4-one
Synonyms: cis-(-)-2-(2-chlorophenyl)-5,7-dihydroxy-8-[4¢-(3¢-hydroxy-1¢-methyl)-piperidinyl]-4H-1-benzopyran-4-one
Molecular Formula: C21H20ClNO5
Molecular Weight: 401.84
Percent Composition: C 62.77%, H 5.02%, Cl 8.82%, N 3.49%, O 19.91%
Literature References: Flavone inhibitor of cyclin-dependent kinases; the (-)-cis form induces apoptosis in certain tumor cells. Prepn: S. L. Kattige
et al., EP 241003;
eidem, US 4900727 (1987, 1990 both to Hoechst). Properties and formulation study: R.-M. Dannenfelser
et al., PDA J. Pharm. Sci. Technol. 50, 356 (1996). Mode of action study: S. Brüsselbach
et al., Int. J. Cancer 77, 146 (1998). Clinical evaluation in refractory cancers: A. M. Senderowicz
et al., J. Clin. Oncol. 16, 2986 (1998). Clinical pharmacokinetics: J. P. Thomas
et al., Cancer Chemother. Pharmacol. 50, 465 (2002). Review of chemistry, pharmacology, and antitumor spectrum: H. H. Sedlacek
et al., Int. J. Oncol. 9, 1143-1168 (1996).
Derivative Type: Hydrochloride
CAS Registry Number: 131740-09-5
Manufacturers' Codes: L-86-8275; HMR-1275; NSC-649890
Molecular Formula: C21H20ClNO5.HCl
Molecular Weight: 438.30
Percent Composition: C 57.55%, H 4.83%, Cl 16.18%, N 3.20%, O 18.25%
Properties: mp 169.5± 0.5° (Dannenfelser). Also reported as yellow powder, mp 188° containing 4.73-6.2% water of crystallization (Sedlacek). pKa 5.68 ± 0.06. [a]24D -1.73 to -3.9°. Soly (mg/ml): water, D5W, and normal saline all <5 at pH 4. Soly (mg/ml): ethanol 10.1; PEG 400 >73.8; propylene glycol >88.1; M-pyrol very much greater than 103.0; t-butyrolactone very much greater than 105.5.
Melting point: mp 169.5± 0.5° (Dannenfelser); mp 188°
pKa: pKa 5.68 ± 0.06
Optical Rotation: [a]24D -1.73 to -3.9°
Therap-Cat: Antineoplastic.
Keywords: Antineoplastic.