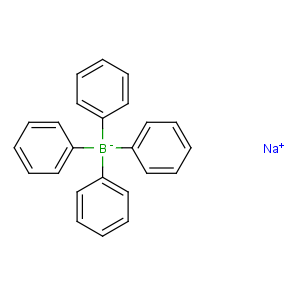
Title: Sodium Tetraphenylborate
CAS Registry Number: 143-66-8
Synonyms: Tetraphenylboron sodium
Trademarks: Kalignost
Molecular Formula: C24H20BNa
Molecular Weight: 342.22
Percent Composition: C 84.23%, H 5.89%, B 3.16%, Na 6.72%
Line Formula: Na[B(C6H5)4]
Literature References: Prepn: Wittig, Raff,
Ann. 573, 195 (1950);
US 2853525 (1958); Heyl,
GB 705719 (1954); Kozlova, Pal'm,
Zh. Obshch. Khim. 31, 2922 (1961); Holtzapfel, Richter,
ibid. 32, 1358 (1962).
Properties: Snow-white crystals from chloroform. Freely sol in water, acetone. Less sol in ether, chloroform. Practically insol in petr ether. Aq solns should be adjusted to a pH of about 5 and can be stored at room temp or lower. Such solns have been stored at 45° for 5 days without deterioration. The soly in polar solvents increases as the temp decreases.
Use: As reagent for determination of potassium, ammonium, rubidium and cesium ions: Barnard, Buechl,
Chemist-Analyst 48, 44, 49 (1959); Montequi, Serrano,
An. R. Acad. Farm. 26, 107 (1960).