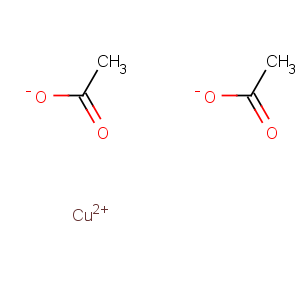
Title: Cupric Acetate
CAS Registry Number: 142-71-2
CAS Name: Acetic acid copper (2+) salt
Molecular Formula: C4H6CuO4
Molecular Weight: 181.63
Percent Composition: C 26.45%, H 3.33%, Cu 34.99%, O 35.24%
Line Formula: Cu(CH3COO)2
Literature References: Prepd by the action of acetic acid on CuO or CuCO3: Winter
et al., in
Kirk-Othmer Encyclopedia of Chemical Technology vol. 6 (Interscience, New York, 2nd ed., 1965) p 267. Toxicity study: H. F. Smyth
et al., Am. Ind. Hyg. Assoc. J. 30, 470 (1969).
Derivative Type: Monohydrate
Synonyms: Neutral verdigris
Properties: Dark green, monoclinic crystals; exists in dimeric units. mp 115°; dec at 240°; d 1.882. Sol in water, alcohol; slightly sol in ether, glycerol. LD50 orally in rats: 0.71 g/kg (Smyth).
Melting point: mp 115°
Density: d 1.882
Toxicity data: LD50 orally in rats: 0.71 g/kg (Smyth)
Derivative Type: Verdigris
CAS Registry Number: 8007-61-2
CAS Name: C.I. Pigment Green 20
Synonyms: C.I. 77408
Properties: Prepn:
Colour Index vol. 4 (3rd ed, 1971) p 4666. Commercial product is a mixture of
basic cupric acetate salts with variable waters of hydration, resulting in blue to green powders. Slightly sol in water, ethanol; sol in dil acids, ammonia.
Use: Intermediate in manuf of Paris green,
q.v.; as fungicide; as catalyst for organic reactions, including rubber aging; in textile dyeing; as pigment for ceramics.