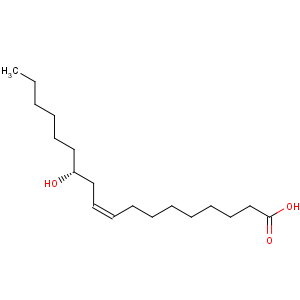
Title: Ricinoleic Acid
CAS Registry Number: 141-22-0
CAS Name: (9
Z,12
R)-12-Hydroxy-9-octadecenoic acid
Synonyms: d-12-hydroxyoleic acid
Molecular Formula: C18H34O3
Molecular Weight: 298.46
Percent Composition: C 72.44%, H 11.48%, O 16.08%
Line Formula: CH3(CH2)5CH(OH)CH2CH=CH(CH2)7COOH
Literature References: Found primarily in oils from the seeds of
Ricinus spp,
Euphorbiaceae. Accounts for about 90% of the triglyceride fatty acids of castor oil, and up to about 40% of the glyceride fatty acids of ergot oil. Bibliography on its isoln: Ralston,
Fatty Acids (New York, 1948) p 189. Also isolated from
Linum mucronatum (flax),
Linaceae: Kleiman, Spencer,
Lipids 6, 962 (1971). Structure: Goldsobel,
Ber. 27, 3121 (1894). Mechanism of biosynthesis: Morris,
Biochem. Biophys. Res. Commun. 29, 311 (1967).
Properties: Liquid. d427.4 0.940; mp 5.5°; bp10 245°. [a]D22 +6.67°; [a]D26 +7.15° (c = 5 in acetone).
nD20 1.4716. Neutralization value 187.98; iodine value 85.05. Sol in alcohol, acetone, ether, chloroform (
cf. the solubilities of castor oil).
Melting point: mp 5.5°
Boiling point: bp10 245°
Optical Rotation: [a]D22 +6.67°; [a]D26 +7.15° (c = 5 in acetone)
Index of refraction: nD20 1.4716
Density: d427.4 0.940
Derivative Type: Acid sulfate
Synonyms: Ricinolsulfuric acid
Molecular Formula: C18H34O6S
Molecular Weight: 378.52
Percent Composition: C 57.12%, H 9.05%, O 25.36%, S 8.47%
Properties: Obtained by the action of chlorosulfonic acid. Viscous brown liquid with weak blue fluorescence. Sol in water (about 10%), alcohol, ether, chloroform.
Derivative Type: Sodium salt
CAS Registry Number: 5323-95-5
Trademarks: Soricin; Colidosan
Properties: Sodium salts of the fatty acids from castor oil. White or slightly yellow, odorless or almost odorless powder. Sol in water or alcohol. The aq soln is alkaline.
Use: In textile finishing; sometimes added to Turkey red oil, dry-cleaning soaps.
Therap-Cat: Has been used in contraceptive jellies. The sodium salt has been used as sclerosing agent.