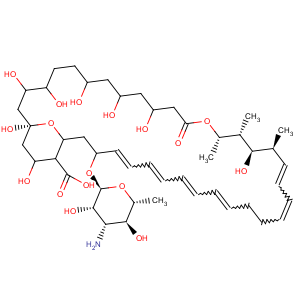
Title: Nystatin
CAS Registry Number: 1400-61-9
Synonyms: Fungicidin
Trademarks: Biofanal (Pfleger); Diastatin; Candex (Miles); Candio-Hermal (Hermal); Mycostatin (BMS); Moronal (BMS); Nystan (BMS); O-V Statin (BMS)
Literature References: Polyene antifungal antibiotic complex containing 3 biologically active components, A1, A2, A3. Produced by
Streptomyces noursei, S. aureus and other
Streptomyces spp: Hazen, Brown,
Science 112, 423 (1950);
Proc. Soc. Exp. Biol. Med. 76, 93 (1951); Raubitscheck
et al., Antibiot. Chemother. 2, 179 (1952); Cohen, Webb,
Arch. Pediatr. 69, 414 (1952); Dutcher
et al., Antibiot. Annu. 1953-1954, 191;
eidem, Therapy of Fungus Diseases (Little, Brown, Boston, 1955) p 168. Review of early literature: Brown, Hazen,
Trans. N.Y. Acad. Sci. 19 (1956-1957) pp 447-456. Purification: Vandeputte,
US 2832719 (1958 to Olin Mathieson); Renella,
US 3517100 (1970 to Am. Cyanamid). Chemistry and partial structure: Birch
et al., Tetrahedron Lett. 1964, 1491; of nystatin A1 and A2: Shenin
et al., Antibiotiki 13, 387 (1968). Structure of the aglycone: Manwaring
et al., ibid. 1969, 5319. Complete structure of A1: Chong, Rickards,
ibid. 1970, 5145; Borowski
et al., ibid. 1971, 685. Revised structure: R. C. Pandey, K. L. Rinehart,
J. Antibiot. 29, 1035 (1976). Stereochemical study of A1: J. M. Lancelin
et al., Tetrahedron Lett. 29, 2827 (1988). Structure of A3: J. Zielinski
et al., J. Antibiot. 41, 1289 (1988). Mechanism of action: R. W. Holz in
Antibiotics vol. 5, pt. 2, F. E. Hahn, Ed. (Springer-Verlag, New York, 1979) pp 313-340. Toxicity study: H. Seneca,
Antibiot. Annu. 1955-1956, 697. Comprehensive description: G. W. Michel,
Anal. Profiles Drug Subs. 6, 341-421 (1977).
Properties: Light yellow powder. Gradually decomposes above 160° without melting by 250°. [a]D25 -10° (glacial acetic acid); +21° (pyridine); +12° (DMF); -7° (0.1
N HCl in methanol). uv max (ethanol): 290, 307, 322 nm. Exhibits strong reducing properties. Solubilities determined by Weiss
et al., Antibiot. Chemother. 7, 374 (1957) in mg/ml at about 28°: water 4.0; methanol 11.2; ethanol 1.2; carbon tetrachloride 1.23; chloroform 0.48; benzene 0.28; ethylene glycol 8.75. Solns and aq suspensions begin to lose activity soon after prepn. Aq suspensions are stable for 10 minutes on heating to 100° at pH 7.0; also stable in moderately alkaline media, but labile at pH 9 and pH 2. Heat, light, and oxygen accelerate decompn. Activity not diminished by blood or serum. LD50 i.p. in mice: ~200 mg/kg (Seneca).
Optical Rotation: [a]D25 -10° (glacial acetic acid); +21° (pyridine); +12° (DMF); -7° (0.1
N HCl in methanol)
Absorption maximum: uv max (ethanol): 290, 307, 322 nm
Toxicity data: LD50 i.p. in mice: ~200 mg/kg (Seneca)
Derivative Type: Nystatin A1
CAS Registry Number: 34786-70-4
Molecular Formula: C47H75NO17
Molecular Weight: 926.09
Percent Composition: C 60.96%, H 8.16%, N 1.51%, O 29.37%
Therap-Cat: Antifungal.
Therap-Cat-Vet: Antifungal; growth promotant.
Keywords: Antifungal (Antibiotics); Polyenes.