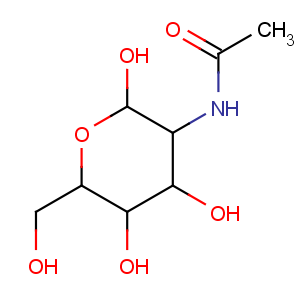
Title: Chitin
CAS Registry Number: 1398-61-4
Literature References: (C8H13NO5)
n. C 47.29%, H 6.45%, N 6.89%, O 39.37%. Cellulose-like biopolymer consisting predominantly of unbranched chains of b-(1?4)-2-acetamido-2-deoxy-D-glucose (also named
N-acetyl-D-glucosamine) residues. Found in fungi, yeasts, marine invertebrates and arthropods, where it is a principal component in the exoskeletons. May be regarded as a derivative of cellulose, in which the C-2 hydroxyl groups have been replaced by acetamido residues. Occurrence in lower animals: A. G. Richards,
The Integument of Arthropods (University of Minnesota Press, Minneapolis, 1951). Occurrence in the plant kingdom: F. von Wettstein,
Handbuch der systematischen Botanik (F. Deuticke, Leipzig and Vienna, 4th ed., 1933). Typical isoln: Hackman,
Aust. J. Biol. Sci. 7, 168 (1954); Horowitz
et al., J. Am. Chem. Soc. 79, 5046 (1957). Occurrence in the anthozoan
Stylobates aeneus Dall, the first sea anemone proved capable of synthesizing chitin: D. F. Dunn, M. H. Liberman,
Science 221, 157 (1983). Structure: Dweltz,
Biochim. Biophys. Acta 44, 416 (1960);
51, 283 (1961); Carlstrom,
ibid. 59, 361 (1962); Ramachadran, Ramakrishman,
ibid. 63, 307 (1962).
Review: Foster, Webber,
Adv. Carbohydr. Chem. 15, 371-393 (1960); C. Jeuniaux, "Chitinous Structures" in
Comprehensive Biochemistry vol. 26c, M. Florkin, E. H. Stotz, Eds. (Elsevier, New York, 1971) pp 595-632. Book: R. A. A. Muzzarelli,
Chitin (Pergamon Press, New York, 1977). Review of properties and possible novel applications: P. R. Austin
et al., Science 212, 749-753 (1981).
Properties: Amorphous solid. Practically insol in water, dil acids, dil and concd alkalies, alcohol and other organic solvents; sol in concd HCl, H2SO4, 78-97% H3PO4, anhydr HCOOH. There are substantial variations in solubility, molecular weight, acetyl values, specific rotation among chitins of different origins and prepared by different methods.
Derivative Type: Acetate
Literature References: Prepn: Shorigin, Hait,
Ber. 68, 971 (1935).
Properties: Sol in HCOOH, 50% resorcinol, concd HCl, H2SO4, HNO3; practically insol in organic solvents.
Derivative Type: Sulfate
Literature References: Process for sulfating chitin: Cushing, Kratovil,
US 2755275 (1956 to Abbott). Sulfated chitin has been found to possess anticoagulant properties in laboratory animals: Roth
et al., Am. J. Physiol. 171, 761 (1952);
Proc. Soc. Exp. Biol. Med. 86, 315 (1954).
Use: Deacylated chitin,
chitosan, used in water treatment; in photographic emulsions; in improving the dyeability of synthetic fibers and fabrics.
Therap-Cat: Vulnerary.
Keywords: Vulnerary.