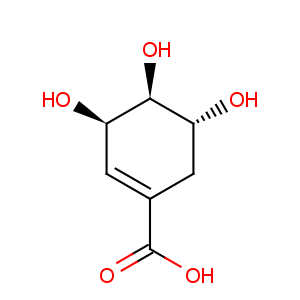
Title: Shikimic Acid
CAS Registry Number: 138-59-0
CAS Name: [3
R-(3a,4a,5b)]-3,4,5-Trihydroxy-1-cyclohexene-1-carboxylic acid
Molecular Formula: C7H10O5
Molecular Weight: 174.15
Percent Composition: C 48.28%, H 5.79%, O 45.94%
Literature References: Naturally occurring (-)-form is a major biosynthetic precursor of phenylalanine, tyrosine, and tryptophan and hence of the majority of plant alkaloids. It is also involved in the biosynthesis of lignin,
q.v., flavonoids and other important aromatic compounds. Isoln from the fruit of the oriental plant
Illicium religiosum Sieb. et Zucc.,
Magnoliaceae (called in Japanese
shikimi-no-ki): J. F. Eykman,
Rec. Trav. Chim. 4, 32 (1885);
idem, Ber. 24, 1278 (1891). Structural study: H. O. L. Fischer, G. Dangshat,
Helv. Chim. Acta 17, 1200 (1934). Configuration:
eidem, ibid. 18, 1206 (1935);
20, 705 (1937). Conformation in soln: L. D. Hall,
J. Org. Chem. 29, 297 (1964). Enzymatic synthesis: P. R. Srinivasan
et al., J. Am. Chem. Soc. 77, 4943 (1955). Stereospecific synthesis: R. McCrindle
et al., J. Chem. Soc. 1960, 1560; E. E. Smissman
et al., J. Am. Chem. Soc. 84, 1040 (1962). Improved synthesis: J. L. Pawlak, G. A. Berchtold,
J. Org. Chem. 52, 1765 (1987). Synthesis of the (±)-form: R. Grewe, I. Hinrichs,
Ber. 97, 443 (1964); R. Grewe, S. Kersten,
Angew. Chem. 77, 859 (1965). Carcinogenicity study: I. A. Evans, M. A. Osman,
Nature 250, 348 (1974).
Reviews: B. A. Bohm,
Chem. Rev. 65, 435-466 (1965); B. Ganem,
Tetrahedron 34, 3353-3383 (1978); J. B. Harborne,
Biosynthesis 6, 40-75 (1980). Books: E. Haslam,
The Shikimate Pathway (Halsted Press, New York, 1974) 316 pp;
idem, "Shikimic Acid Metabolites" in
Comprehensive Organic Chemistry vol. 5 (Pergamon Press, Oxford, 1979) pp 1167-1205; U. Weiss, J. M. Edwards,
The Biosynthesis of Organic Compounds (Wiley, New York, 1980) 728 pp.
Properties: Needles from methanol/ethyl acetate, mp 183-184.5°. Sublimes. [a]D -161° (c = 0.57 in methanol). [a]D18 -183.8° (c = 4.03 in water). uv max (alcohol): 213 nm (e 8900). pK (14.1°) 5.19. Soly in water about 18 g/100 ml. Soly at 23° (g/100 m): 2.25 in abs alcohol: 0.015 in anhydr ether. Practically insol in chloroform, benzene, petr ether. LD5 i.p. in mice: 1000 mg/kg (Evans, Osman).
Melting point: mp 183-184.5°
pKa: pK (14.1°) 5.19
Optical Rotation: [a]D -161° (c = 0.57 in methanol); [a]D18 -183.8° (c = 4.03 in water)
Absorption maximum: uv max (alcohol): 213 nm (e 8900)
Toxicity data: LD5 i.p. in mice: 1000 mg/kg (Evans, Osman)
Derivative Type: (±)-Form
Properties: Needles from methanol/ethyl acetate, mp 191-192°. uv max (ethanol): 212 nm (e 8200).
Melting point: mp 191-192°
Absorption maximum: uv max (ethanol): 212 nm (e 8200)