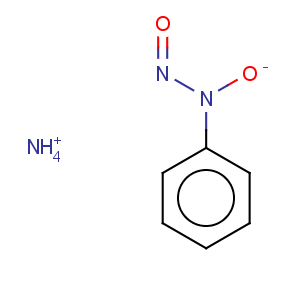
Title: Cupferron
CAS Registry Number: 135-20-6
CAS Name: N-Hydroxy-
N-nitrosobenzenamine ammonium salt
Synonyms: N-nitrosophenylhydroxylamine ammonium salt
Molecular Formula: C6H9N3O2
Molecular Weight: 155.15
Percent Composition: C 46.45%, H 5.85%, N 27.08%, O 20.62%
Literature References: Prepd from phenylhydroxylamine by treating with NaNO2 at 0° in presence of HCl, filtering, dissolving in ether and treating with NH3, or by treating an ether soln of phenylhydroxylamine with butyl or amyl nitrite and NH3 in the cold: Marvel, Kamm,
Org. Synth. 4, 19 (1925).
Properties: Crystals, mp 163-164°. Freely sol in water or alc.
Keep in well-closed containers to which a piece of ammonium carbonate has been added.
Melting point: mp 163-164°
CAUTION: This substance is reasonably anticipated to be a human carcinogen:
Report on Carcinogens, Eleventh Edition (PB2005-104914, 2004) p III-73.
Use: As a reagent for separating Sn from Zn, and Cu and Fe from other metals. Ppts iron quantitatively from strongly acid soln; as a quantitative reagent for vanadates with which it gives a dark-red ppt sol in alkali soln, and for Ti with which it forms a yellow ppt; also suitable for the colorimetric estimation of Al.