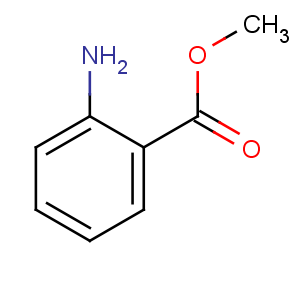
Title: Methyl Anthranilate
CAS Registry Number: 134-20-3
CAS Name: 2-Aminobenzoic acid methyl ester
Synonyms: methyl 2-aminobenzoate; neroli oil (artificial)
Molecular Formula: C8H9NO2
Molecular Weight: 151.16
Percent Composition: C 63.57%, H 6.00%, N 9.27%, O 21.17%
Literature References: Occurs in neroli, ylang-ylang, bergamot, jasmine, other essential oils and in grape juice; also obtained synthetically by esterifying anthranilic acid with CH3OH in presence of HCl.
Properties: Crystals. d 1.168. mp 24-25°. bp15 135.5°. Slightly sol in water; freely sol in alcohol or ether. LD50 orally in rats, mice: 2910, 3900 mg/kg, P. M. Jenner
et al., Food Cosmet. Toxicol. 2, 327 (1964).
Melting point: mp 24-25°
Boiling point: bp15 135.5°
Density: d 1.168
Toxicity data: LD50 orally in rats, mice: 2910, 3900 mg/kg, P. M. Jenner
et al., Food Cosmet. Toxicol. 2, 327 (1964)
Use: As perfume for ointments; manuf synthetic perfumes.