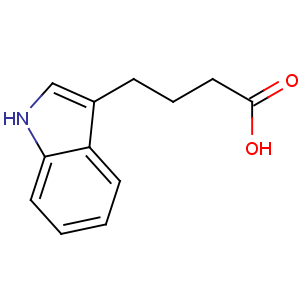
Title: Indolebutyric Acid
CAS Registry Number: 133-32-4
CAS Name: 1
H-Indole-3-butanoic acid
Synonyms: indole-3-butyric acid; 4-(3-indolyl)butyric acid
Trademarks: Seradix (Rhone-Poulenc)
Molecular Formula: C12H13NO2
Molecular Weight: 203.24
Percent Composition: C 70.92%, H 6.45%, N 6.89%, O 15.74%
Literature References: Prepn: Jackson, Manske,
J. Am. Chem. Soc. 52, 5029 (1930); by heating indole, g-butyrolactone, and sodium hydroxide, followed by acidification of the product: Fritz,
US 3051723 (1962 to Union Carbide); by decarboxylation of 2-carboxyindole-3-butyric acid: Bowman, Islip,
Chem. Ind. (London) 1971, 154. Toxicity studies: Anderson
et al., Proc. Soc. Exp. Biol. Med. 34, 138 (1936); Pesonen,
Acta Endocrinol. 5, 409 (1950). Hypoglycemic effect in rats: Mirsky
et al., Endocrinology 59, 715 (1956).
Properties: White or slightly yellow crystals. Slight characteristic odor. mp 123-125°. Practically insol in water, chloroform. Sol in alc, ether, acetone. LD50 i.p. in mice: 100 mg/kg (Anderson).
Melting point: mp 123-125°
Toxicity data: LD50 i.p. in mice: 100 mg/kg (Anderson)
Use: To stimulate root formation of plant clippings.