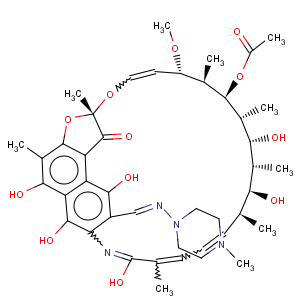
Title: Rifampin
CAS Registry Number: 13292-46-1
CAS Name: 3-[[(4-Methyl-1-piperazinyl)imino]methyl]rifamycin
Synonyms: 5,6,9,17,19,21-hexahydroxy-23-methoxy-2,4,12,16,18,20,22-heptamethyl-8-[
N-(4-methyl-1-piperazinyl)formimidoyl]-2,7-(epoxypentadeca[1,11,13]trienimino)naphtho[2,1-
b]furan-1,11(2
H)-dione 21-acetate; rifampicin; rifaldazine; rifamycin AMP; R/AMP
Trademarks: Abrifam (Abbott); Eremfat (Fatol); Rifa (Grñenthal); Rifadin(e) (Aventis); Rifaldin (Aventis); Rifapiam (Piam); Rifaprodin (Almirall); Rifoldin (Aventis); Rimactan(e) (Novartis)
Molecular Formula: C43H58N4O12
Molecular Weight: 822.94
Percent Composition: C 62.76%, H 7.10%, N 6.81%, O 23.33%
Literature References: Semisynthetic antibiotic obtained by reacting 3-formylrifamycin SV with 1-amino-4-methylpiperazine in tetrahydrofuran. Prepn and structure: Maggi
et al., Chemotherapia 11, 285 (1966);
NL 6509961; Maggi, Sensi,
US 3342810 (1966, 1967 both to Lepetit). Chemical and biological properties: Fürész,
Antibiot. Chemother. 16, 316 (1970). Activity studies and clinical survey: Arioli
et al., Arzneim.-Forsch. 17, 523 (1967); Pallanza
et al., ibid. 529; Bergamini,
ibid. 20, 1546 (1970); Dans
et al., Am. J. Med. Sci. 259, 120 (1970). Metabolism: Meyer-Brunot
et al., Int. Congr. Chemother. Proc., 5th, Vienna 1967 1(2), 763; Fürész
et al., Arzneim.-Forsch. 17, 534 (1967); Maggi
et al., ibid. 19, 651 (1969). Inhibition of protein synthesis in mammalian cells: W. C. Buss
et al., Science 200, 432 (1978). Comprehensive reviews: Binda
et al., Arzneim.-Forsch. 21, 1907-1978 (1971); Lester,
Annu. Rev. Microbiol. 26, 88-102 (1972). Comprehensive description: G. G. Gallo, P. Radaelli,
Anal. Profiles Drug Subs. 5, 467-513 (1976). Symposium on the use of rifampin in the treatment of nontuberculous infections:
Rev. Infect. Dis. 5, Suppl. 3, S399-S632 (1983).
Properties: Red to orange platelets from acetone, dec 183-188°. Absorption max (pH 7.38): 237, 255, 334, 475 nm (e 33200, 32100, 27000, 15400). Rifampin is a "zwitterion" with pKa 1.7 related to the 4-hydroxy and pKa 7.9 related to the 3-piperazine nitrogen. Very stable in DMSO; rather stable in water. Freely sol in CH3Cl, DMSO; sol in ethyl acetate, methanol, tetrahydrofuran; slightly sol in water (pH <6), acetone, carbon tetrachloride. LD50 in mice, rats (mg/kg): 885, 1720 orally; 260, 330 i.v.; 640, 550 i.p. (Fürész).
pKa: pKa 1.7 related to the 4-hydroxy and pKa 7.9 related to the 3-piperazine nitrogen
Absorption maximum: Absorption max (pH 7.38): 237, 255, 334, 475 nm (e 33200, 32100, 27000, 15400)
Toxicity data: LD50 in mice, rats (mg/kg): 885, 1720 orally; 260, 330 i.v.; 640, 550 i.p. (Fürész)
Therap-Cat: Antibacterial (tuberculostatic).
Keywords: Antibacterial (Antibiotics); Ansamycins; Antibacterial (Tuberculostatic).