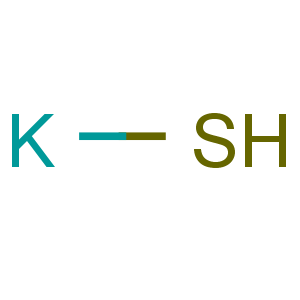Title: Potassium Bisulfide
CAS Registry Number: 1310-61-8
Synonyms: Potassium hydrosulfide; potassium hydrogen sulfide; potassium sulfhydrate
Molecular Formula: HKS
Molecular Weight: 72.17
Percent Composition: H 1.40%, K 54.18%, S 44.43%
Line Formula: KHS
Literature References: Prepd industrially from Ca(HS)2 and K2SO4: Hene,
DE 380385 (1922); from H2S and K2S: Bassett,
US 1662735 (1925); Strosacker, Jones,
US 1771384 (1926 to Dow). Prepn of pure material by the action of dry H2S upon potassium metal dissolved in abs ethanol: Rule,
J. Chem. Soc. 99, 558, 564 (1911); West,
Z. Kristallogr. 88, 102 (1934).
Properties: Colorless, deliquescent crystals or white, strongly hygroscopic, cryst mass. Usually present as the hemihydrate. Trigonal system. d 1.70. Rapidly becomes yellow on exposure to air with formation of polysulfides and H2S. Becomes anhydr at 175-200°. mp 450-510° forming a dark red liquid. Heat of formation: +62.5 kcal. Heat of soln at 17°: +0.77 kcal, for hemihydrate at 16°: +0.62 kcal. Freely sol in water, alcohol.
Melting point: mp 450-510° forming a dark red liquid
Density: d 1.70