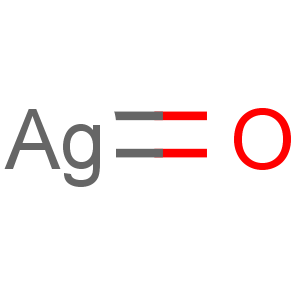Title: Silver(II) Oxide
CAS Registry Number: 1301-96-8
Synonyms: Argentic oxide; silver peroxide; silver suboxide
Trademarks: Divasil
Molecular Formula: AgO
Molecular Weight: 123.87
Percent Composition: Ag 87.08%, O 12.92%
Literature References: Is actually a silver(I)-silver(III) oxide: McMillan,
Chem. Rev. 62, 65 (1962). Prepd by the oxidation of silver nitrate with potassium peroxydisulfate in an alk medium: Hammer, Kleinberg,
Inorg. Synth. 4, 12 (1953). Prepn of single crystals:
Chem. Eng. News 47, 32 (Aug 11, 1969).
Properties: Charcoal-gray powder.
Avoid contact with skin, organic matter, strong ammonia and alkalies. Malleable. Cubic or orthorhombic system when cryst. d425 7.483. The dry solid is stable at 100° for 18 hrs, dec above 100° into silver and oxygen. Possesses semiconductor properties and is diamagnetic.
Strong oxidizing agent. Ka 7.9 ′ 10-13. Practically insol in water: 27 mg/l at 25° (decompn). Sol in alkalies and NH4OH (with decompn and evolution of N2). In dil acids oxygen is evolved immediately, in concd acid intensely colored solns are formed (brown in nitric acid and olive green in sulfuric acid).
Density: d425 7.483
CAUTION: Highly irritating to skin, eyes, mucous membranes, respiratory tract.
Use: In the manuf of silver oxide-zinc alkali batteries.