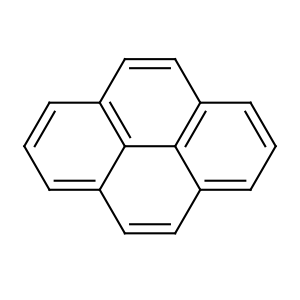
Title: Pyrene
CAS Registry Number: 129-00-0
Synonyms: Benzo[
def]phenanthrene
Molecular Formula: C16H10
Molecular Weight: 202.25
Percent Composition: C 95.02%, H 4.98%
Literature References: Occurs in coal tar,
q.v. Isoln: Kruber,
Ber. 64, 84 (1931). Also obtained by the destructive hydrogenation of hard coal: I. G. Farben.,
DE 639240;
DE 640580;
DE 654201. Purification by chromatography: Winterstein
et al., Z. Physiol. Chem. 230, 162 (1934). Synthesis from
o,o¢-ditolyl: Weitzenb?ck,
Monatsh. Chem. 34, 193 (1913). From
peri-trimethylenenaphthalene and malonyl chloride with AlCl3: Fleischer, Retze,
Ber. 55, 3280 (1922). From a-tetralone: Braun, Rath,
Ber. 61, 956 (1928). From 4-keto-1,2,3,4-tetrahydrophenanthrene by a Reformatsky reaction: Cook, Hewett,
J. Chem. Soc. 1934, 366. Review of toxicology and human exposure:
Toxicological Profile for Polycyclic Aromatic Hydrocarbons (PB95-264370, 1995) 487 pp.
Properties: Monoclinic prismatic tablets from alcohol or by sublimation. d23 1.271. Pure pyrene is colorless, the usual contaminant which gives it a yellow color is tetracene. Solid and solns have slight blue fluorescence. mp 156°. bp 404°. Absorption spectrum: Clar,
Ber. 69, 1677 (1936); Seshan,
Proc. Indian Acad. Sci. A3, 148 (1936). Fluorescence maxima: Sannié, Poremski,
Bull. Soc. Chim. [5]
3, 1139 (1936). Insol in water. Fairly sol in organic solvents.
Melting point: mp 156°
Boiling point: bp 404°
Density: d23 1.271