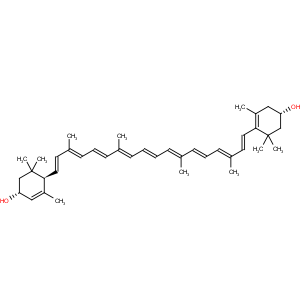
Title: Xanthophyll
CAS Registry Number: 127-40-2
CAS Name: b,e-Carotene-3,3¢-diol
Synonyms: lutein; vegetable lutein; vegetable luteol
Trademarks: Bo-Xan
Molecular Formula: C40H56O2
Molecular Weight: 568.87
Percent Composition: C 84.45%, H 9.92%, O 5.62%
Literature References: One of the most widespread carotenoid alcohols in nature. Originally isolated from egg yolk, also isolated by chromatography from nettles, algae, and petals of many yellow flowers. Occurs also in colored feathers of birds: Volker,
Z. Physiol. Chem. 288, 20 (1951). Extraction from petals of
Tagetes patula L.,
Compositae: Karrer
et al., Helv. Chim. Acta 30, 531 (1947). Occurs together with zeaxanthin,
q.v. Dipalmitate occurs in
Helenium autumnale L.,
Compositae and other flowers: Kuhn, Winterstein,
Naturwissenschaften 18, 754 (1930). Conversion to zeaxanthin with sodium alcoholate: Karrer, Jucker,
ibid. 266. Does not possess vitamin A potency: Schumacher
et al., Poult. Sci. 23, 529 (1944). Stereochemistry: Zechmeister,
Chem. Rev. 34, 267 (1944). Structure: Karrer,
Helv. Chim. Acta 34, 2160 (1951). Abs config: Goodfellow
et al., Chem. Commun. 1970, 1578; Buchecker
et al., Chimia 25, 192 (1971);
eidem, Helv. Chim. Acta 57, 631 (1974). Synthesis: H. Mayer, A. Rüttimann,
ibid. 63, 1451 (1980). Sepn and determn of configurational isomers: A. Rüttiman
et al., J. High Resolut. Chromatogr. Chromatogr. Commun. 6, 612 (1983).
Reviews: Zechmeister,
Carotinoide (Berlin, 1934); Mayer,
The Chemistry of Natural Coloring Matters (New York, 1943); Karrer, Jucker,
Carotenoids (New York, 1950).
Properties: Yellow prisms with metallic luster from ether + methanol, mp 190° (corr), (a higher mp indicates impure material). Also reported as mp 183° [Buchecker (1974)]. [a]18Cd +165° (c = 0.7 in benzene). Absorption max (dioxane): 481, 453, 429, 333, 268 nm (e 142000, 152000, 100000, 15500, 35000). Insol in water, sol in fats and in fat solvents. More sol in boiling methanol (1:700) than zeaxanthin.
Melting point: Yellow prisms with metallic luster from ether + methanol, mp 190° (corr), (a higher mp indicates impure material); mp 183° [Buchecker (1974)]
Optical Rotation: [a]18Cd +165° (c = 0.7 in benzene)
Absorption maximum: Absorption max (dioxane): 481, 453, 429, 333, 268 nm (e 142000, 152000, 100000, 15500, 35000)
Derivative Type: Dipalmitate
Synonyms: Helenien
Trademarks: Adaptinol (Bayer)
Molecular Formula: C72H116O4
Molecular Weight: 1045.69
Percent Composition: C 82.70%, H 11.18%, O 6.12%
Properties: Red needles from alcohol, mp 92°.
Melting point: mp 92°