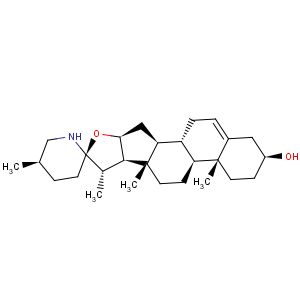
Title: Solasodine
CAS Registry Number: 126-17-0
CAS Name: (3b,22a,25
R)-Spirosol-5-en-3-ol
Synonyms: solasod-5-en-3b-ol; D5-20bF,22aF,25aF,27-azaspirosten-3b-ol; solancarpidine; solanidine-S; purapuridine
Molecular Formula: C27H43NO2
Molecular Weight: 413.64
Percent Composition: C 78.40%, H 10.48%, N 3.39%, O 7.74%
Literature References: Steroidal alkaloid isolated from various
Solanum species. By hydrolysis of solasonine: Rochelmeyer,
Arch. Pharm. 277, 329 (1939).
See also ref
under Solasonine and Solanidine. Structure: Briggs
et al., J. Chem. Soc. 1950, 3013. Synthesis: Uhle,
J. Org. Chem. 27, 656 (1962); Schreiber, R?nsch,
Tetrahedron 20, 1939 (1964); Kessar
et al., ibid. 27, 2869 (1971). Comprehensive description: G. Indrayanto
et al., Anal. Profiles Drug Subs. Excip. 24, 487-522 (1996).
Properties: Hexagonal plates from methanol or by sublimation in high vacuum, mp 200-202°. [a]D25 -98° (c = 0.14 in methanol); [a]D -113° (CHCl3). Alkaline reaction to litmus in alcoholic soln. pKb 6.30. uv max (methanol): 206 nm. Freely sol in benzene, pyridine, and chloroform. Practically insol in ether. Soly at 30° (mg/ml): methanol 9.5; 95% ethanol 5.0; acetone 3.5;
n-hexane <1.0; water <1.0.
Melting point: mp 200-202°
pKa: pKb 6.30
Optical Rotation: [a]D25 -98° (c = 0.14 in methanol); [a]D -113° (CHCl3)
Absorption maximum: uv max (methanol): 206 nm
Use: Starting material for steroidal drugs.